Abstract
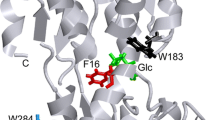
To gain further insight into the difference in substrate specificity between endoglucanase and cellobiohydrolase, the intrinsic fluorescence properties of cellobiohydrolase I (CBH I) and endoglucanase I (EG I) from Trichoderma pseudokiningii S-38 were investigated. The results for the spectral characteristics, ligand binding and fluorescence quenching suggest that the fluorescence of two enzymes comes from tryptophan residues, and that tryptophan residue(s) may be involved in the function of the two enzymes. The results also suggest that the binding tryptophan in EG I may be more exposed to solvent than that in CBH I. This interpretation is supported by the observations that the effects of pH upon the fluorescence of EG I are greater than that of CBH I; spectral shifts are different in EG I and CBH I under various conditions, and fluorescence lifetime changes caused by cellobiose binding are larger for EG I than for CBH I.
Similar content being viewed by others
REFERENCES
Claeyssens, M., van Tilberugh, H., Tomme, P., Wood, T. M., and Mcpae, S. T. (1989). Biochem. J. 261, 819–825.
Damude, H., Ferro, V., Withers, S. G., and Warren, R. A. J. (1996). Biochem J. 315, 467–472.
Divne, C., Stahlberg, J., Reinikainen, T., Rouhonen, L., Pettersson, G., Knowles, J. K. C., Teeri, T. T., and Jones, T. A. (1994). Science 265, 514–528.
Fersht, A. (1985). Enzyme Structure and Mechanism, 2nd ed., Freeman, San Francisco, Chapter 6.
Gilkes, N. R., Henrissat, B., Kilburn, B. G., Miller, R. C. Jr., and Warren, R. A. T. (1991). Microb. Rev. 55, 303–315.
Hahn, M., Olsen, O., Poltz, O., Borriss, R., and Heineman, U. (1995). J. Biol. Chem. 270, 3081–3088.
Juy, M., Amit, A. G., Alzari, R. M., Poljak, R. T., Claeyssens, M., Beguin, P., and Aubert, J. P. (1992). Nature 357, 89–91.
Linder, M., Linderberg, G., Reinikainen, I., Teeri, T. T., and Pettersson, G. (1995). FEBS Lett. 372, 96–98.
Lowry, O. H., Rosenbrough, N. J., Farr, A. L., and Randall, R. L. (1951). J. Biol. Chem. 193, 265–275.
Ma, D. B., Gao, P. J., and Wang, Z. N. (1990). Enzyme Microbial Technol. 12, 631–635.
Meinke, A., Damude, H. G., Tomme, P., Kwan, E., Kilburn, B., Miller, R. C. Jr., Warren, R. A. J., and Gilkes, N. R. (1995) J. Biol. Chem. 270, 4383–4388.
Penttila, M., Lehtovaara, P., Nevalainen, H., Bhikhabhai, R., and Knowles, J. (1986). Gene 45, 253–263.
Rouvinen, J., Bergfors, T., Teeri, T., Knowles., and Jones, T. A. (1990). Science 249, 380–386.
Spezio, M., Wilson, D. B., and Karplus, P. A. (1993). Biochemistry 32, 9906–9916.
Teale, F. W., and Weber, G. (1957). Biochem. J. 65, 476–482.
Van Tilberugh, H., Pettersson, G., Bhikabhai, R., Boek, H., and Claeyssens, M. (1985). Eur. J. Biochem. 148, 329–334.
White, A., Handler, P., Smith, E. L., Hill, R. L., and Lehman, I. R. (1978). Principles of Biochemistry, 6th ed., McGraw-Hill, New York.
White, A., Withers, S. G., Gilkes, N. R., and Rose, D. R. (1994) Biochemistry 33, 12546–12552.
Woodward, J., Lee, N. E., Carmicheal, J. S., Macnair, S. L., and Wichert, J. M. (1990). Biochim. Biophys. Acta 1037, 81–85.
Yan, B. X., and Gao, P. J. (n.d.). Chin. Biochem J., in press.
Yan, B. X., and Sun, Y. Q. (1997). J. Protein Chem. 16, 59–66.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Xu, Y.B., Qing, S.Y. & PeiJi, G. Intrinsic Fluorescence in Endoglucanase and Cellobiohydrolase from Trichoderma pseudokiningii S-38: Effects of pH, Quenching Agents, and Ligand Binding. J Protein Chem 16, 681–688 (1997). https://doi.org/10.1023/A:1026354403952
Published:
Issue Date:
DOI: https://doi.org/10.1023/A:1026354403952