Abstract
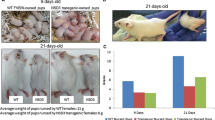
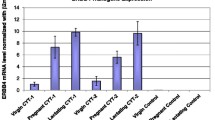
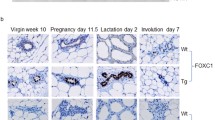
Transforming growth factor-alpha (TGFα)4 and/or the EGF receptor (EGFR) are frequently overexpressed by human and rodent breast tumors, as well as tumor-derived cell lines. Additionally, various observations suggest a role for TGFα and the EGFR signaling system in normal mouse mammary gland development. Recently, several laboratories have established TGFα transgenic mice with which to study the role of this growth factor in normal and neoplastic mammary biology. Examination of these mice revealed that overexpression of TGFα has profound consequences for this tissue. Most strikingly, transgenic mice expressing TGFα under the control of tissue-specific and nonspecific promoters stochastically developed focal mammary tumors with an incidence and latency that was markedly affected by pregnancy. Most TGFα-induced tumors were well-differentiated adenomas/adenocarcinomas, although some were undifferentiated and locally invasive. Distant metastases were only occasionally observed. Administration of the genotoxic carcinogen, 7,12-dimethylbenzanthracene (DMBA), dramatically accelerated mammary tumorigenesis induced by the TGFα transgene, raising the possibility that TGFα acts as a promoter in this tissue. Mice harboring dual transgenes encoding TGFα and either wild-type ERBB2 or c-myc displayed markedly accelerated tumorigenesis compared to mice carrying any of the single transgenes alone, indicative of potent cooperativity. Moreover, tumorigenesis in the bitransgenic mice was less dependent on pregnancy, and tumors were generally more malignant in appearance. Finally, TGFα also affected mammary gland dynamics. TGFα transgenic mice consistently displayed precocious alveolar development, were variably impaired with respect to lactation, and showed markedly reduced postlactional involution. As a result, the glands of multiparous females accumulated hyperplastic lesions that generally resembled milk-producing alveoli. Limited data support the hypothesis that these lesions were precursors to TGFα-induced tumors. In summary, these various findings underscore the potential importance of TGFα for cellular differentiation and transformation in the mammary gland. They also establish TGFα transgenic mice as a powerful model with which to study the role of EGFR signaling molecules in this dynamic tissue.
Similar content being viewed by others
REFERENCES
D. C. Lee, S. E. Fenton, E. A. Berkowitz, and M. A. Hissong (1995). Transforming growth factor α: expression, regulation, and biological activities. Pharmacol. Rev. 47:51–85.
N. C. Luetteke, T. H. Qiu, R. L. Peiffer, P. Oliver, O. Smithies, and D. C. Lee (1993). TGFα deficiency results in hair follicle and eye abnormalities in targeted and waved-1 mice. Cell 73:263–278.
G. B. Mann, K. J. Fowler, A. Gabriel, E. C. Nice, R. L. Williams, and A. R. Dunn (1993). Mice with a null mutation of the TGFα gene have abnormal skin architecture, wavy hair, and curly whiskers and often develop corneal inflammation. Cell 73:249–261.
E. A. Berkowitz, K. B. Seroogy, J. A. Schroeder, W. E. Russell, E. P. Evans, R. F. Riedel, H. K. Phillips, C. A. Harrison, D. C. Lee, and N. C. Luetteke (1996). Characterization of the mouse transforming growth factor α gene: its expression during eyelid development and in waved I tissues. Cell Growth Diff. 7:1271–1282.
L. Panico, A. D'Antonio, G. Salvatore, E. Mezza, G. Tortora, M. D. Laurentiis, S. D. Placido, T. Giordano, M. Merino, D. S. Salomon, W. J. Mullick, G. Pettinato, S. J. Schnitt, A. R. Bianco, and F. Ciardiello (1996). Differential immunohistochemical detection of transforming growth factor alpha, amphiregulin and CRIPTO in human normal and malignant breast tissues. Int. J. Cancer. 65:51–56.
R. B. Dickson, M. D. Johnson, M. Bano, E. Shi, J. Kurebayashi, B. Ziff, I. Martinez-Lacaci, L. T. Amundadottir, and M. E. Lippman (1992). Growth factors in breast cancer: mitogenesis to transformation. J. Steroid Biochem. Mol. Biol. 43:69–78.
K. Stromberg, M. Duffy, C. Fritsch, W. R. Hudgins, E. S. Sharp, L. D. Murphy, M. E. Lippman, and S. E. Bates (1991). Comparison of urinary transforming growth factor-alpha in women with disseminated breast cancer and healthy control women. Cancer Det. Prev. 15:277–283.
S. C. Liu, B. Sanfillipo, I. Perroteau, R. Derynck, D. S. Salomon, and W. R. Kidwell (1987). Expression of transforming growth factor α (TGFα) in differentiated rat mammary tumors: estrogen induction of TGFα production. Mol. Endocrinol. 1:683–692.
S. E. Bates, N. E. Davidson, E. M. Valverius, C. E. Freter, R. B. Dickson, J. P. Tam, J. E. Kudlow, M. E. Lippman, and D. S. Salomon (1988). Expression of transforming growth factor α and its messenger ribonucleic acid in human breast cancer: its regulation by estrogen and its possible functional significance. Mol. Endocrinol. 2:543–555.
C. L. Arteaga, E. Coronado, and C. K. Osborne (1988). Blockade of the epidermal growth factor receptor inhibits transforming growth factor α-induced but not estrogen-induced growth of hormone-dependent human breast cancer. Mol. Endocrinol. 2:1064–1069.
M. L. McGeady, S. Kerby, V. Shankar, F. Ciardiello, D. Salomon, and M. Seidman (1989). Infection with a TGF-α retroviral vector transforms normal mouse mammary epithelial cells but not normal rat fibroblasts. Oncogene 4:1375–1382.
A. Rosenthal, P. B. Lindquist, T. S. Bringman, D. V. Goeddel, and R. Derynck (1986). Expression in rat fibroblasts of a human transforming growth factor-α cDNA results in transformation. Cell 46:301–309.
E. P. Sandgren, N. C. Luetteke, R. D. Palmiter, R. L. Brinster, and D. C. Lee (1990). Overexpression of TGFα in transgenic mice: induction of epithelial hyperplasia, pancreatic metaplasia, and carcinoma of the breast. Cell 61:1121–1135.
C. Jhappan, C. Stahle, R. N. Harkins, N. Fausto, G. H. Smith, and G. T. Merlino (1990). TGFα overexpression in transgenic mice induces liver neoplasia and abnormal development of the mammary gland and pancreas. Cell 61:1137–1146.
P. J. Dempsey, J. R. Goldenring, C. J. Soroka, I. M. Modlin, R. W. McClure, C. D. Lind, A. D. Ahlquist, M. R. Pittelkow, D. C. Lee, E. P. Sandgren, D. L. Page, and R. J. Coffey (1992). Possible role of transforming growth factor α in the patholgenesis of Menetrier's disease: supportive evidence from humans and transgenic mice. Gastroenterology. 103:1950–1963.
H. Takagi, C. Jhappan, R. Sharp, and G. Merlino (1992). Hypertrophic gastropathy resembling menetrier's disease in transgenic mice overexpressing transforming growth factor α in the stomach. J. Clin. Invest. 90:1161–1167.
Y. Matsui, S. A. Halter, J. T. Holt, B. L. M. Hogan, and R. J. Coffey (1990). Development of mammary hyperplasia and neoplasia in MMTV-TGFα transgenic mice. Cell 61:1147–1155.
S. A. Halter, P. Dempsey, Y. Matsui, M. K. Stokes, R. Graves-Deal, B. L. Hogan, and R. J. Coffey (1992). Distinctive patterns of hyperplasia in transgenic mice with mouse mammary virus transforming growth factor-α. Amer. J. Path. 140:1131–1146.
E. P. Sandgren, J. A. Schroeder, T. H. Qui, R. D. Palmiter, R. L. Brinster, and D. C. Lee (1995). Inhibition of mammary gland involution is associated with transforming growth factor α but not c-myc-induced tumorigenesis in transgenic mice. Cancer Res. 55:3915–3927.
R. A. McKnight, T. Burdon, V. G. Pursel, A. Shamay, R. J. Wall, and L. Hennighausen (1991). Genes, oncogenes, and hormones: advances in cellular and molecular biology of breast cancer, In The Whey Acidic Protein. R. B. Dickson and M. E. Lippman, (eds.), Kluwer Academic Publishers, Boston, pp 399–412.
G. H. Smith, R. Sharp, E. C. Kordon, C. Jhappan, and G. Merlino (1995). Transforming growth factor-α promotes mammary tumorigenesis through selective survival and growth of secretory epithelial cells. Am. J. Pathol. 147:1081–1096.
M. Hollstein, D. Sidransky, B. Vogelstein, and C. Harris (1991). p53 mutations in human cancers. Washington D.C. Science 253:49–53.
H. Matsushime, M. F. Roussel, R. A. Ashmun, and C. J. Sherr (1991). Colony-stimulating factor 1 regulates novel cyclins during the G1 phase of the cell cycle. Cell 65:701–713.
C. L. Rosenberg, T. Motokura, H. M. Kronenberg, and A. Arnold (1993). Coding sequence of the overexpressed transcript of the putative oncogene PRAD1/cylin D1 in two primary human tumors. Oncogene 8:519–521.
E. Schuuring, E. Verhoeven, W. J. Mooi, and R. J. Michalides (1992). Identification and cloning of two overexpressed genes, U21B3/PRAD1 and EMS1, within the amplified chromosome 11q13 region in human carcinomas. Oncogene 7:355–361.
C. Gillet, V. Fantl, R. Smith, C. Fisher, J. Bartek, C. Dickson, D. Barnes, and G. Peters (1994). Amplification and overexpression of cyclin D1 in breast cancer detected by immunohistochemical staining. Cancer Res. 54:1812–1817.
W. Jiang, S. M. Kahn, N. Tomita, Y.-J. Zhang, S.-H. Lu, and I. B. Weinstein (1992). Amplification and expression of the human cyclin D gene in esophageal cancer. Cancer Res. 52:2980–2983.
T. C. Wang, R. D. Cardiff, L. Zukerberg, E. Lees, A. Arnold, and E. V. Schmidt (1994). Mammary hyperplasia and carcinoma in MMTV-cyclin D1 transgenic mice. (London) Nature 369:669–671.
A. Philipp, A. Schneider, I. Vasrik, K. Finke, Y. Xiong, D. Beach, K. Alitalo, and M. Eilers (1994). Repression of cyclin D1: a novel function of myc. Mol. Cell. Biol. 14:4032–4043.
L. T. Amundadottir, M. D. Johnson, G. Merlino, G. H. Smith, and R. B. Dickson (1995). Synergistic interaction of transforming growth factor α and c-myc in mouse mammary and salivary gland tumorigenesis. Cell Growth Diff. 6:737–748.
S. Sakai, M. Mizuno, T. Harigaya, K. Yamamoto, T. Mori, R. J. Coffey, and H. Nagasawa (1994). Cause of failure of lactation in mouse mammary tumor virus/human transforming growth factor α transgenic mice. Proc. Soc. Exp. Biol. Med. 205:236–242.
R. Strange, G. Li, S. Saure, A. Burkhardt, and R. R. Friis (1992). Apoptotic cell death and tissue remodeling during mouse mammary gland involution. Development 115:49–58.
D. Medina (1982). The mouse in biomedical research: experimental biology and oncology. In H. L. Foster, J. D. Small, and J. G. Fox, (eds.) Academic Press, New York, pp. 373–396.
M. Reiss, E. B. Stash, V. F. Vellucci, and Z.-L. Zhou (1991). Activation of the autocrine transforming growth factor α pathway in human squamous carcinoma cells. Cancer Res. 51:6254–6262.
E. Finzi, T. Ho, G. Anhalt, W. Hawkins, R. Harkins, and T. Horn (1992). Localization of transforming growth factor-α in human appendageal tumors. Am. J. Pathol. 141:643–653.
C. Liu, M.-S. Tsao, and J. W. Grisham (1988). Transforming growth factors produced by normal and neoplastically transformed rat liver epithelia cells in culture. Cancer Res. 48:850–855.
C. Liu, A. Woo, and M.-S. Tsao (1990). Expression of transforming growth factor-alpha in primary human colon and lung carcinomas. Br. J. Cancer 62:425–429.
J. W. Grisham, M.-S. Tsao, D. C. Lee, and H. S. Earp (1990). Sequential changes in epidermal growth factor receptor/ligand function in cultured rat liver epithelial cells transformed chemically in vitro. Pathobiology 58:3–14.
L. W. Lee, V. W. Raymond, M.-S. Tsao, D. C. Lee, H. S. Earp, and J. W. Grisham (1991). Clonal cosegregation of tumorigenicity with overexpression of c-myc and transforming growth factor-α genes in chemically transformed rat liver epithelial cells. Cancer Res. 51:5238–5244.
S. Tanaka, K. Imanishi, M. Yoshihara, K. Haruma, K. Sumii, G. Kajiyama, and S. Akamatsu (1991). Immunoreactive transforming growth factor alpha is commonly present in colorectal neoplasia. Am. J. Pathol. 139:123–129.
R. J. Coffey Jr., K. S. Meise, Y. Matsui, B. L. M. Hogan, P. J. Dempsey, and S. A. Halter (1994). Acceleration of mammary neoplasia in transforming growth factor α transgenic mice by 7,12-dimethylbenzanthracene. Cancer Res. 54:1678–1683.
W. E. Russell, W. K. Kaufmann, S. Sitaric, N. C. Luetteke, and D. C. Lee (1996). Liver regeneration and hepatocarcinogenesis in transforming growth factor-α-targeted mice. Mol. Carcin. 15:183–189.
J. M. Varley, J. E. Swallow, W. J. Brammar, J. L. Whittaker, and R. A. Walker (1987). Alterations to eithr c-erbB-2(neu) or c-myc and proto-oncogenes in breast carcinomas correlate with poor short-term prognosis. Oncogene 1:423–430.
R. B. Dickson, A. Kasid, K. K. Huff, S. E. Bates, C. Knabbe, D. Bronzert, E. P. Gelmann, and M. E. Lippman (1987). Activation of growth factor secretion in tumorigenic states of breast cancer induced by 17β-estradiol or v-Ha-ras oncogene. Proc. Natl. Acad. Sci. U.S.A. 84:837–841.
R. B. Dickson, K. K. Huff, E. M. Spencer, and M. E. Lippman (1986). Induction of epidermal growth factor-related polypeptides by 17β-estradiol in MCF-7 human breast cancer cells. Endocrinology 118:138–142.
S. E. Bates, M. E. McManaway, M. E. Lippman, and R. B. Dickson (1986). Characterization of estrogen responsive transforming activity in human breast cancer cell lines. Cancer Res. 46:1707–1713.
D. Dubik, T. C. Dembinski, and R. P. C. Shiu (1987). Stimulation of c-myc oncogene expression associated with estrogen-induced proliferation of human breast cancer cell. Cancer Res. 47:6517–6521.
D. Dubik and R. P. C. Shiu (1988). Transcriptional regulation of c-myc oncogene expression by estrogen in homone-responsive human breast cancer cells. J. Biol. Chem. 263:12705–12708.
E. P. Sandgren, N. C. Luetteke, T. H. Qiu, R. D. Palmiter, R. L. Brinster, and D. C. Lee (1993). Transforming growth factor alpha dramatically enhances oncogene-induced carcinogenesis in transgenic mouse pancreas and liver. Mol. Cell. Biol. 13:320–330.
C. R. King, M. H. Kraus, and S. A. Aaronson (1985). Amplification of a novel v-erbB related gene in human mammary carcinoma. Science 229:974–976.
D. J. Slamon, G. M. Clark, S. G. Wong, W. J. Levin, A. Ullrich, and W. L. McGuire (1987). Human breast cancer: correlation of relapse and survival with amplification of Her-2/neu oncogene. Science 235:177–182.
M. van de Vijver, R. van de Bersselaar, P. Devilee, C. Cornelisse, J. Peterse, and R. Nusse (1987). Amplification of the neu (c-erbB-2) oncogene in human mammary tumors is relatevely frequent and is often accompanied by amplification of the linked c-erbA oncogene. Mol. Cell Biol. 7:2019–2023.
W. J. Gullick, S. B. Love, C. Wright, D. M. Barnes, B. Gutterson, A. L. Harris, and D. G. Altman (1991). c-erbB-2 protein overexpression in breast cancer is a risk factor in patients with involved and uninvolved lymph nodes. Br. J. Cancer. 63:434–438.
M. C. Paterson, K. D. Dietrich, J. Danyluk, A. H. Paterson, A. W. Lees, N. Jamil, J. Hanson, H. Jenkins, B. E. Krause, W. A. McBlain, D. J. Slamon, and R. M. Fourney (1991). Correlation between c-erbB-2 amplification and risk of recurrent disease in node-negative breast cancer. Cancer Res. 51:556–567.
E. Tzahar, H. Waterman, X. Chen, G. Levkowitz, D. Karunagaran, S. Lavi, B. J. Ratzkin, and Y. Yarden (1996). A hierarchical network of interreceptor interactions determines signal transduction by neu differentiation factor/neuregulin and epidermal growth factor. Mol. Cell. Biol. 16:5276–5287.
W. J. Muller, E. Sinn, R. Wallace, P. K. Pattengale, and P. Leder (1988). Single-step induction of mammary adenocarcinoma in transgenic mice bearing the activated c-neu oncogene. Cell 54:105–115.
L. Bouchard, L. Lamarre, P. J. Tremblay, and P. Jolicoeur (1989). Stochastic appearance of mammary tumors in transgenic mice carrying the activated c-neu oncogene. Cell 57:931–936.
C. T. Guy, M. A. Webster, M. Schaller, T. J. Parson, R. D. Cardiff, and W. J. Muller (1992). Expression of the neu proto-oncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc. Natl. Acad. Sci. U.S.A. 89:10578–10582.
P. M. Siegel, D. L. Dankort, W. R. Hardy, and W. J. Muller (1994). Novel acting mutations in the neu proto-oncogene involved in induction of mammary tumors. Mol. Cell Biol. 14:7068–7077.
W. J. Muller, C. L. Arteaga, S. K. Muthuswamy, P. M. Siegel, M. A. Webster, R. D. Cardiff, K. S. Meise, F. Li, S. A. Halter, and R. J. Coffey (1996). Synergistic interaction of the neu proto-oncogene product and transforming growth factor α in the mammary epithelium of transgenic mice. Mol. Cell Biol. 16:5726–5736.
D. S. Liscia, G. Merlo, F. Ciardello, N. Kim, G. H. Smith, R. Callahan, and D. S. Salomon (1990). Transforming growth factor-α messenger RNA localization in the developing adult rat and human mammary gland by in situ hybridization. Develop Biol. 140:123–131.
S. M. Snedeker, C. F. Brown, and R. P. Augustine (1991). Expression and functional properties of transforming growth factor α and epidermal growth factor during mouse mammary gland ductal morphogenesis. Proc. Natl. Acad. Sci. U.S.A. 88:8167–8171.
N. C. Luetteke, H. K. Phillips, T. H. Qiu, N. G. Copeland, H. S. Earp, N. A. Jenkins, and D. C. Lee (1994). The mouse waved-2 phenotype results from a point mutation in the EGF receptor tyrosine kinase. Genes and Dev. 8:263–278.
K. J. Fowler, F. Walker, W. Alexander, M. L. Hibbs, E. C. Nice, R. M. Bohmer, G. B. Mann, C. Thumwood, R. Maglitto, J. A. Danks, R. Chetty, A. W. Burgess, and A. R. Dunn (1995). A mutation in the epidermal growth factor receptor in waved-2 mice has a profound effect on receptor biochemistry that results in impaired lactation. Proc. Natl. Acad. Sci. U.S.A. 92:1465–1469.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schroeder, J.A., Lee, D.C. Transgenic Mice Reveal Roles for TGFα and EGF Receptor in Mammary Gland Development and Neoplasia. J Mammary Gland Biol Neoplasia 2, 119–129 (1997). https://doi.org/10.1023/A:1026347629876
Issue Date:
DOI: https://doi.org/10.1023/A:1026347629876