Abstract
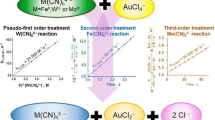
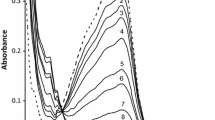
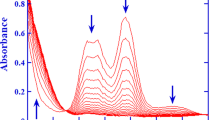
The oxidation of H2NOH is first-order both in [NH3OH+] and [AuCl4 −]. The rate is increased by the increase in [Cl−] and decreased with increase in [H+]. The stoichiometry ratio, Δ[NH3OH+]/Δ[AuCl4 −], is ≈1. The mechanism consists of the following reactions.
The rate law deduced from the reactions (i)–(iv) is given by Equation (v) considering that [H+] ≫ K a.
The reaction (iii) is a combination of the following reactions:
The activation parameters for the reactions (ii) and (iii) are consistent with an outer-sphere electron transfer mechanism.
Similar content being viewed by others
References
K.K. Sengupta and B. Basu, Transition Met. Chem., 8, 6 (1983).
A.I. Vogel, A Textbook of Quantitative Inorganic Analysis, Longmans, London, 1986, p. 391.
Ms Rekha, A. Prakash and R.N. Mehrotra, Can. J. Chem., 71, 2164 (1991).
K.K. Sengupta, A. Sanyal and P.K. Sen, Transition Met. Chem., 19, 534 (1994).
F.H. Fry, G.A. Hamilton and J. Turkevich, Inorg. Chem., 5, 1943 (1966).
P.V.Z. Bekker and W. Robb, Inorg. Chim. Acta, 8, 849 (1972).
B.S. Maritz and R. Van Eldik, Inorg. Chim. Acta, 17, 21 (1976).
B.I. Peshchevitskii, V.I. Belevantsev and N.V. Kuvatova, Russ. J. Inorg. Chem. (Engl. Transl.), 16, 1007 (1971).
S. Dholiya, A. Prakash and R.N. Mehrotra, J. Chem. Soc., Dalton Trans., 819 (1992).
B.S. Maritz and R. Van Eldik, J. Inorg. Nucl. Chem., 38, 1749 (1976).
B.S. Maritz and R. Van Eldik, J. Inorg. Nucl. Chem., 19, 1035 (1977).
B.S. Maritz and R. Van Eldik, J. Inorg. Nucl. Chem., 38, 1545 (1976).
W.J. Louw and W. Robb, Inorg. Chim. Acta, 3, 303 (1969).
K.G. Moodley and M.J. Nicol, J. Chem. Soc. Dalton Trans., 993 (1977).
W. Robb. Inorg. Chem., 6, 382 (1967).
G. Natile, E. Bordignonand and L. Cattalini, Inorg. Chem., 15, 246 (1976).
H.G. Forsberg, B. Widdel and L.G. Erwall, J. Chem. Educ., 37, 44 (1960).
B. Goyal, A. Prakash and R.N. Mehrotra, Indian J. Chem., Sect. A, 38, 541 (1999).
R.N. Mehrotra and L.J. Kirschenbaum, Inorg. Chem., 28, 4327 (1989).
I. Kouadio, L.J. Kirschenbaum and R.N. Mehrotra, J. Chem. Soc., Dalton Trans., 1929 (1990).
I. Kouadio, L.J. Kirschenbaum, R.N. Mehrotra and Y. Sun, J. Chem. Soc., Dalton Trans., 2123 (1990).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Soni, V., Mehrotra, R.N. Kinetics and mechanism of oxidation of hydroxylamine by tetrachloroaurate(III) ion. Transition Metal Chemistry 28, 893–898 (2003). https://doi.org/10.1023/A:1026330125535
Issue Date:
DOI: https://doi.org/10.1023/A:1026330125535