Abstract
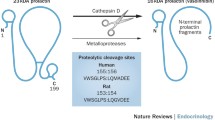
An enzymatically cleaved form of rat prolactin (rPRL) was first described in 1980. This cleavage produces a molecule that consists of two chains of amino acids linked by a disulfide bond between two Cys residues. Reduction of that bond produces two fragments of 6 and 16 Kd. A considerable amount of information has accrued in recent years about the cleaved molecule and its 16-Kd fragment. The enzyme that cleaves the molecule is present in target tissues of PRL in rodents (e.g., mammary gland and ventral prostate), and the activity of the enzyme changes with the functional state of the mammary gland. Rat mammary PRL-cleaving activity is specific for rodent PRL, and the enzyme is localized in the stroma of that gland. The enzyme that cleaves rPRL is probably cathepsin D, and the sites of cleavage on the molecule have been identified. The cleaved form of rPRL has a high degree of activity in various assays, but it has reduced activity in radioimmunoassays. The 16-Kd fragment retains a significant degree of bioactivity in in vitro mitogenic assays, and specific binding sites for the fragment have been identified. Novel bioactivities for the cleaved form of rPRL and its 16-Kd fragment have been reported, and these molecules may account for the fact that bioassay estimates of PRL in rat serum are generally higher than are RIA measurements. Although the 16-Kd fragment has significant bioactivity, it contains only six of the fourteen residues that are thought to participate in the coupling of the intact hormone to its receptor. Cleaved rPRL is present in rat serum (but not in milk), but whether the 16-Kd fragment is formed in vivo has not yet been determined.
Similar content being viewed by others
REFERENCES
O. Riddle (1963). Prolactin in vertebrate function and organization. J. Natl. Cancer Inst. 31:1039–1110.
Y. N. Sinha (1995). Structural variants of prolactin: Occurrence and physiological significance. Endocrinol. Rev. 16:354–369.
I. Mittra (1980). A novel “cleaved prolactin” in the pituitary. I. Biosynthesis, characterization and regulatory control. Biochem. Biophys. Res. Commun. 95:1750–1759.
I. Mittra (1980). A novel “cleaved prolactin” in the rat pituitary. II. In vivo mammary mitogenic activity of its N-terminal 16K moiety. Biochem. Biophys. Res. Commun. 95:1760–1767.
Y. N. Sinha and T. A. Gilligan (1984). A cleaved form of prolactin in the mouse pituitary gland: Identification and comparison of in vitro synthesis and release in strains with high and low incidences of mammary tumors. Endocrinology 114:2046–2053.
M. Andries, D. Tilemans, and C. Denef (1992). Isolation of cleaved prolactin variants that stimulate DNA synthesis in specific cell types in rat pituitary cell aggregates in culture. Biochem. J. 281:393–400.
X. Casabiell, M. C. Robertson, H. G. Friesen, and F. F. Casanueva (1989). Cleaved prolactin and its 16K fragment are generated by an acid protease. Endocrinology 125:1967–1972.
S. Meuris, M. Svoboda, M. Vilamala, J. Christophe, and C. Robyn (1983). Monomeric pituitary growth hormone and prolactin variants in man characterized by immunoperoxidase electrophoresis. FEBS Letters 154:111–115.
Y. N. Sinha, T. A. Gilligan, D. W. Lee, D. Hollingsworth, and E. Markoff (1985). Cleaved prolactin: Evidence for its occurrence in human pituitary gland and plasma. J. Clin. Endocrinol. Metab. 60:239–243.
C. Clapp, P. S. Sears, D. H. Russell, J. Richards, B. K. Levay-Young, and C. S. Nicoll (1988). Biological and immunological characterization of cleaved and 16K forms of rat prolactin. Endocrinology 122:2892–2898.
M. M. Compton and R. J. Witorsch (1983). Proteolytic fragmentation of rat prolactin by the ventral prostate gland. Prostate 4:231–238.
M. M. Compton and R. J. Witorsch (1984). Proteolytic degradation and modification of rat prolactin by subcellular fractions of the rat ventral prostate gland. Endocrinology 115:476–484.
V. L. Y. Wong, M. M. Compton, and R. J. Witorsch (1986). Proteolytic modification of rat prolactin by subcellular fractions of the lactating rat mammary gland. Biochim. Biophys. Acta 881:167–175.
C. Clapp (1987). Analysis of the proteolytic cleavage of prolactin by the mammary gland and liver of the rat: Characterization of the cleaved and 16K forms. Endocrinology 121:2055–2064.
W. J. DeVito, C. Avakian, and S. Stone (1992). Proteolytic modification of prolactin by the female rat brain. Neuroendocrinology 56:597–603.
W. J. DeVito (1988). Heterogeneity of immunoreactive prolactin in the rat brain. Biochem. Biophys. Res. Commun. 150:599–604.
C. Clapp, L. Torner, G. Gutierrez-Ospina, E. Alcantara, F. J. Lopez-Gomez, M. Nagano, P. A. Kelly, S. Mejia, M. A. Morales, and G. Martinez de la Escalera (1994). The prolactin gene is expressed in the hypothalamic-neurohypophyseal system and the protein is processed into a 14-kDa fragment with activity like 16-kDa prolactin. Proc. Nat. Acad. Sci. U.S.A. 91:10384–10388.
L. Torner, S. Mejia, F. J. Lopez-Gomez, A. Quintanar, G. Martinez de la Escalera, and C. Clapp (1995). A 14-kilodalton prolactin-like fragment is secreted by the hypothalamo-neurohypophyseal system of the rat. Endocrinology 136:5454–5460.
R. A. Baldocchi, Y.-C. Chou, and C. S. Nicoll (1994). Cleavage of rat prolactin occurs within rat mammary tissue, and the cleaved form is present in serum but not in milk. PSEBM 206:416–420.
R. A. Baldocchi, L. Tan, and C. S. Nicoll (1992). Processing of rat prolactin by rat tissue explants and serum in vitro. Endocrinology 130:1653–1659.
R. A. Baldocchi, L. Tan, D. S. King, and C. S. Nicoll (1993). Mass spectrometric analysis of the fragments produced by cleavage and reduction of rat prolactin: Evidence that the cleaving enzyme is cathepsin D. Endocrinology 133:935–938.
R. A. Baldocchi, L. Tan, Y. K. Hom, and C. S. Nicoll (1995). Comparison of the ability of normal mouse mammary tissues and mammary adenocarcinoma to cleave rat prolactin. PSEBM 208:283–287.
R. S. Vick, V. L. Y. Wong, and R. J. Witorsch (1987). Biological, immunological and biochemical characterization of cleaved prolactin generated by lactating mammary gland. Biochim. Biophys. Acta 931:196–204.
J. Zull and J. Chuang (1985). Characterization of parathyroid hormone fragments produced by cathepsin D. J. Biol. Chem. 260:1608–1613.
A. J. Barrett and J. K. McDonald (1980). Mammalian Proteases: A Glossary and Bibliography, Vol. 1: Endopeptidases. Academic Press, New York, p. 338.
G. V. Marionchenko and R. P. Khropycheva (1989). The lysosomal pathway of prolactin degradation in the mammary gland: Kinetics of hydrolysis of prolactin by cathepsin D and fragments formed. Biokhimiya 54:629–639.
C. Clapp, P. S. Sears, and C. S. Nicoll (1989). Binding studies with intact rat prolactin and a 16K fragment of the hormone. Endocrinology 125:1054–1059.
U. J. Lewis (1963). Factors that effect the fragmentation of growth hormone and prolactin by hypophyseal proteinases. J. Biol. Chem. 238:3330–3336.
R. Singh, B. Seavey, V. Rice, T. Lindsey, and U. Lewis (1974). Modified forms of human growth hormone with increased biological activities. Endocrinology 94:883–891.
J. Russell, A. B. Schneider, J. Katzhendler, K. Kowalski, and L. M. Sherwood (1979). Modification of human placental lactogen with plasmin. J. Biol. Chem. 254:2296–2301.
C. H. Li (1975). Human pituitary growth hormone: A biologically active hendekakaihekaton peptide fragment corresponding to amino-acid residues 15–125 in the hormone molecule. Proc. Nat. Acad. Sci. U.S.A. 72:3878–3882.
B. A. Doneen, H. A. Bern, and C. H. Li (1977). Biological actions of human somatotrophin and its derivatives on mouse mammary gland and teleost urinary bladder. J. Endocrinol. 73:377–382.
R. Aston and J. Ivanyi (1983). Antigenic, receptor binding and mitogenic activity of proteolytic fragments of human growth hormone. EMBO J. 2:493–494.
J. Russell, L. M. Sherwood, K. Kowalski, and A. B. Schneider (1979). Preparation of a disulfide-linked dimer of human placental lactogen fragment 1–134 with immunologic and biologic activity. Proc. Nat. Acad. Sci. U.S.A. 76:1204–1207.
J. Russell, L. M. Sherwood, K. Kowalski, and A. B. Schneider (1981). Recombinant hormones from fragments of human growth hormone and human placental lactogen. J. Biol. Chem. 256:296–300.
H. Asawaroengchai (1978). Metabolism of purified and secreted rat prolactin in vitro. Horm. Res. 9:151–156.
F. C. Leung, S. M. Russell, and C. S. Nicoll (1978). Relationship between bioassay and radioimmunoassay estimates of prolactin in rat serum. Endocrinology 103:1619–1628.
H. Asawaroengchai, S. M. Russell, and C. S. Nicoll (1978). Electrophoretically separable forms of rat prolactin with different bioassay and radioimmunoassay activities. Endocrinology 102:407–414.
C. A. Powers (1993). Anterior pituitary glandular kallikrein: A putative prolactin processing protease. Mol. Cell. Endocrinol. 90:c15–c20.
V. Goffin, K. T. Shiverick, P. A. Kelly, and J. A. Martial (1996). Sequence-function relationships within the expanding family of prolactin, growth hormone, placental lactogen, and related proteins in mammals. Endocrinol. Rev. 17:385–410.
C. S. Nicoll, G. L. Mayer, and S. M. Russell (1986). Structural features of prolactins and growth hormones that can be related to their biological properties. Endocrinol. Rev. 7:169–203.
M. Andries, G. F. M. Jacobs, D. Tilemans, and C. Denef (1996). In vitro immunoneutralization of a cleaved prolactin variant: Evidence for a local paracrine action of cleaved prolactin in the development of gonadotrophs and thyrotrophs in rat pituitary. J. Neuroendocrinol. 8:123–127.
N. Ferrara, C. Clapp, and R. Weiner (1991). The 16K fragment of prolactin specifically inhibits basal or fibroblast growth factor stimulated growth of capillary endothelial cells. Endocrinology 129:896–900.
C. Clapp and R. Weiner (1992). A specific, high affinity, saturable binding site for the 16-kilodalton fragment of prolactin on capillary endothelial cells. Endocrinology 130:1380–1386.
M. L. Torner, C. S. Nicoll, and C. Clapp (1991). Identification of 16K prolactin in the serum of rats and mice. Progr. Endocr. Soc. U.S. (Abstract 735), p. 214.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nicoll, C.S. Cleavage of Prolactin by Its Target Organs and the Possible Significance of this Process. J Mammary Gland Biol Neoplasia 2, 81–89 (1997). https://doi.org/10.1023/A:1026329731268
Issue Date:
DOI: https://doi.org/10.1023/A:1026329731268