Abstract
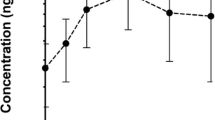
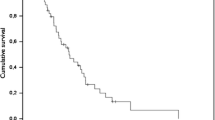
Purpose: To compare two different infusion schedules of phenylacetate (PA) in patients with primary brain tumors and to assess the feasibility of the administration in a multi-institutional setting. Patients and methods: Adult patients with recurrent primary brain tumors were treated with PA on two different schedules. The first schedule (I) consisted of a 2-week continuous, intravenous infusion followed by a 2-week rest period (14 days on, 14 days off) at a dose of 400 mg/kg/day based on ideal body weight. This was repeated once and the 8-week period defined as a cycle. The second schedule (II) consisted of a 12-day continuous infusion at a dose of 400 mg/kg/day based on IBW with a 2-day rest period. This was repeated four times for a duration of 8 weeks which defined one cycle of therapy. Cycles were repeated until tumor progression, unacceptable toxicity, or a delay of more than 28 days from the last day of the preceding infusion. Tumor response was assessed every 8 weeks. The National Cancer Institute toxicity criteria were used to assess toxicity. Dose adjustments were specified for toxicities. Plasma concentrations achieved during the patients' first cycle of therapy were assessed. Results: The clinical results of the phase II study of patients treated on schedule I were previously reported [8]. Of the nine eligible patients treated on schedule II, seven were assessable for radiographic response. There were no objective responses. One patient had stable disease and six had progressive disease. The median survival was 9 months (95% confidence intervals of 3–12 months). The steady state plasma concentrations of PA and phenylacetylglutamine were comparatively the same between the two dosing schedules. However a 1.7-fold greater amount of PA was delivered by schedule II. Conclusion: The infusions were well tolerated. Despite the feasibility of administering this agent in an outpatient setting, there were no responses seen with the more intensive schedule of PA.
Similar content being viewed by others
References
Brusilow SW, Danney M, Waber LJ, Batshaw M, Burton B, Levitsky L, Roth K, McKeethren C, Ward J: Treatment of episodic hyperammonemia in children with inborn errors of urea synthesis. N Engl J Med 310: 1630–1634, 1984
Simell O, Sipila I, Rajantie J, Valle DL, Brusilow SW: Waste nitrogen excretion via amino acid acylation: benzoate and phenylacetate in lysinuric protein intolerance. Pediatr Res 20: 1117–1121, 1986
Watson AJ, Karp JE, Walker WG, Chambers T, Risch VR, Brusilow SW: Transient idiopathic hyperammonaemia in adults. Lancet 2: 1271–1274, 1985
Hudgins WR, Skack S, Myers CE, Samid D: Cytostatic activity of phenylacetate and derivatives against tumor cells. Biochem Pharmacol 50: 1273–1279, 1995
Schmidt F, Groscurth P, Kermer M, Dichgans J, Weller M: Lovastatin and phenylacetate induce apoptosis, but not differentiation, in human malignant glioma cells. Acta Neuropathol 101: 217–224, 2001
Pineau T, Hudgins W, Liu L, Chen L, Sher T, Gonzalez F, Samid D: Activation of a human peroxisome proliferator-activated receptor by the antitumor agent phenylacetate and its analogs. Biochem Pharmacol 52: 659–667, 1996
Ram Z, Samid D, Walbridge S, Oshiro E, Viola J, Tao-Cheng J, Shack S, Thibault A, Myers C, Oldfield E: Growth inhibition, tumor maturation, and extended survival in experimental brain tumors in rats treated with phenylacetate. Cancer Res 54: 2934–2927, 1994
Chang S, Kuhn J, Robins I, Schold SC, Spence AM, Berger MS, Mehta MP, Bozik ME, Pollack I, Schiff D, Gilbert M, Rankin C, Prados M: Phase II study of Phenylacetate in patients with recurrent malignant glioma: A North American brain tumor consortium. J Clin Onc 17: 984–990, 1999
Thibault A, Cooper MR, Figg WD, Venzon DJ, Sartor AO, Tompkins AC, Weinberger MS, Headlee DJ, McCall NA, Samid D, Myers CE: A phase I and pharmacokinetic study of intravenous phenylacetate in patients with cancer. Cancer Res 54: 1690–1694, 1994
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chang, S.M., Kuhn, J.G., Ian Robins, H. et al. A study of a different dose-intense infusion schedule of phenylacetate in patients with recurrent primary brain tumors. Invest New Drugs 21, 429–433 (2003). https://doi.org/10.1023/A:1026299118067
Issue Date:
DOI: https://doi.org/10.1023/A:1026299118067