Abstract
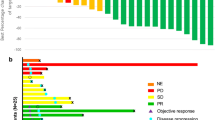
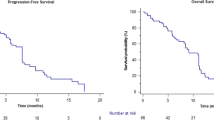
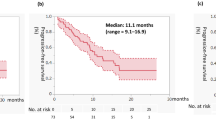
Objective: Rebeccamycin analog (NSC-655649) is an antibiotic with antitumor properties demonstrated in preclinical and phase I studies. We conducted a phase II trial to evaluate the efficacy and toxicity of this agent in patients with advanced renal cell cancer (RCC). Methods: Eligible patients had histologically or cytologically confirmed diagnosis of RCC that was either locally advanced unresectable, locally recurrent, or metastatic. Patients had to have measurable disease, no prior chemotherapy, life expectancy of greater than 12 weeks, an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2, adequate-organ function, and be ≥18 years old. Patients were treated with NSC-655649 at a dose of 165 mg/m2 daily i.v. over 30–60 min for 5 days. Treatment was repeated every 21 days. Response was assessed every two courses. Results: Twenty-four patients were enrolled. There were sixteen males and eight females with a median age of 60.5 years (range 42–76). Nineteen were Caucasians, seventeen had prior nephrectomy, and thirteen had prior immunotherapy. The major toxicity was myelosuppression with grade 3 and 4 neutropenia in 38% of patients and anemia in 33% of patients. There were two partial responses (2/24, 8%) and 11 patients (46%) achieved stable disease (SD). The 6-month progression-free rate for patients with SD was 30%. Of the seventeen patients with progressive disease at registration, one had a PR and eight had SD. The overall median survival time for all 24 patients was 10.0 months (90% CI=5.2, 17.4 months). The 12-month survival rate was 39%, with 90% CI=(0.21, 0.58). Nine patients are still alive with survival times ranging from 3.8 to 24.2 months, at a median follow-up time of 11.9 months. Conclusion: Rebeccamycin analog (NSC-655649) is well tolerated and has modest antitumor activity in patients with advanced RCC.
Similar content being viewed by others
References
Motzer RJ, Mazumdar M, Bacik J, et al.: Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol 17(18): 2530–2540
Bukowski RM: Natural history and therapy of metastatic renal cell carcinoma. The role of interleukin-2. Cancer 80: 1198–1220, 1997
Kaneko T, Wong H, Utzig J, Schurig J, Doyle T: Water soluble derivatives of rebeccamycin. J Antibiot (Tokyo) 43(1): 125–127, 1990
Krishnan BS, Moore ME, Lavoie CP, Long BH, Dalterio RA, Wong HS, Rosenberg IE: Fluorescence polarization studies of the binding of BMS 181176 to DNA. J Biomol Struct Dyn 12(3): 625–636, 1994
Merchant J, Tutsch K, Dresen A, Arzoomanian R, Alberti D, Feierabend C, Binger K, Marnoccha R, Thomas J, Cleary J, Wilding G: Phase I clinical and pharmacokinetic study of NSC 655649, a rebeccamycin analogue, given in both single-dose and multiple-dose formats. Clin Cancer Res 8(7): 2193–2201, 2002
Tolcher AW, Eckhardt SG, Kuhn J, Hammond L, Weiss G, Rizzo J, Aylesworth C, Hidalgo M, Patnaik A et al.: Phase I and pharmacokinetic study of NSC 655649, a rebeccamycin analog with topoisomerase inhibitory properties. J Clin Oncol 19(11): 2937–2947, 2001
Dowlati A, Hoppel CL, Ingalls ST, Majka S, Li X, Sedransk N, Spiro T et al.: Phase I clinical and pharmacokinetic study of rebeccamycin analog NSC 655649 given daily for five consecutive days. J Clin Oncol 19(8): 2309–2318, 2001
Fleming TR: One-sample multiple testing procedure for Phase II clinical trials. Biometrics 38: 143–151, 1982
Casella G: Refining binomial confidence intervals. Canadian J Statst 14: 113–129, 1987
Mehta C, Patel N: StatXact 5: Statistical Software for Exact Nonparametric Inference, User Manual. Cytel Software Corporation, Cambridge, MA 1999, pp. 429–433
Lee ET: In: Statistical Methods for Survival Data Analysis, 2nd edn. New York, Wiley & Sons Inc., 1992, pp. 77–78
Minasian LM, Motzer RJ, Gluck L et al.: Interferone alfa-2a in advanced RCC: treatment results and survival in 159 patients with long-term follow-up. J Clin Oncol 11: 1368–1375, 1993
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hussain, M., Vaishampayan, U., Heilbrun, L.K. et al. A phase II study of rebeccamycin analog (NSC-655649) in metastatic renal cell cancer. Invest New Drugs 21, 465–471 (2003). https://doi.org/10.1023/A:1026259503954
Issue Date:
DOI: https://doi.org/10.1023/A:1026259503954