Abstract
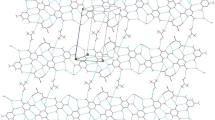
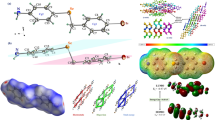
Benzenehexacarboxylic acid, mellitic acid (MA), has been used as a core motif to study possible radial self-assembly using complementary aromatic bases. By mixing water solutions of the components, crystals of the salts of MA with 4-aminopyridine (AP), 4-dimethylamino-pyridine (DM), 2,2′-bipyridine (DP), o-phenanthroline (PL), and melamine (ML) have been obtained. The MA−n ions have assembled in either extended sheets for MA−2 or extended ribbons for MA−4 by direct hydrogen bonding between MA and MA and additionally through mediation of hydrogen bonds to water molecules that distribute the negative charges throughout the MA sheet or ribbon. Most of the O atoms in carboxyl groups in the MA ions in the five complexes have been rotated significantly out of the plane of the central benzene ring. There are multiple base molecules, two or four, for each mellitic acid ion in the five complexes. Most of the NH+ moieties in all five bases make direct NH+ ⋅ ⋅ ⋅ O–C hydrogen bonds with MA−n. The planar base ions are generally arranged in stacks in which the components range from being parallel, with interplanar separations of 3.5 Å, to having a considerable tilt with respect to each other with nearest interplanar separation of atoms greater than 3.9 Å. These geometric characteristics are reflected in the color of the crystals. The three-dimensional networking makes some of the crystals very hard. Cell dimensions: 1, C32H30N8O12 ⋅ 2H2O, C2/c, a =13.764(2) Å, b =18.053(3) Å, c =14.876(4) Å, β =105.99(2)° 2, C26H26N4O12 ⋅ 3H2O, P21/n, a =15.891(1) Å, b =10.444(1) Å, c =18.242(1) Å, β =97.00(1); 3, C64H44N8O24 ⋅7H2O, P21/c, a =23.016(4) Å, b =15.241(2) Å, c =19.124(2) Å, β =100.60(1)° 4, C36H22N4O12, P21/n, a =14.581(1) Å, b =10.472(1) Å, c =20.607(2) Å, β =106.43(1); 5, C18H18N12O12 ⋅ 2H2O, \(P\bar 1\), a =8.257(2) Å, b =8.986(2) Å, c =9.383(1) Å, α =98.60(1)°, β =96.38(2)°, γ =117.07(1)°.
Similar content being viewed by others
References
Lindoy, L.F.; Atkinson, I. M. Self-Assembly in Supramolecular Systems; Royal Society of Chemistry Publication, Springer Verlage, New York, 2000.
Lehn, J.M. Supramolecular Chemistry: Concepts and Perspectives; VCH: Winheim, Germany, 1995.
Reinhoudt, D.N., Ed. Comprehensive Supramolecular Chemistry, Vol. 10; Pergamon: Oxford, 1996.
Ungewickell, E. Curr. Biol. 1999, 9, R 32.
Payne, G.S. J. Membr. Biol. 1990, 116, 93.
Keen, J.H. Annu. Rev. Biochem. 1990, 59, 415.
Brodsky, F.M. Science 1988, 242, 1396.
Wibaut, J.P.; Overhoff, J.; Jonker, E. W.; Gratama, K. Rec. Trav. Chim. 1941, 60, 742.
Darlow, S.F. Acta Cryst. 1961, 14, 159.
Sim, G.A.; Robertson, J.M.; Goodwin, T.H. Acta Cryst. 1955, 8, 157.
Herbstein, F.H. Perspecl. Struc. Chem. 1971, 4, 166.
Kistenmacher, T.J.; Phillips, T.E.; Cowan, D.O. Acta Cryst. 1974, B30, 763.
Kistenmacher, T.J.; Emge, T.J.; Bloch, A.N.; Cowan, D.O. Acta Cryst. 1982, B38, 1193.
Bender, M.L.; Chow, Y.L. J. Am. Chem. Soc. 1959, 81, 392.
Mathias, J.P.; Simanek, E.E.; Zerkowski, J.A.; Seto, Ch. T.; Whitesides, G.M. J. Am. Chem. Soc. 1994, 116, 4316.
Zerkowski, J.A.; Whitesides, G.M. J. Am. Chem. Soc. 1994, 116, 4298.
Janczak, J.; Kubiak, R. Acta Chem. Scand. 1999, 53, 602.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karle, I., Gilardi, R.D., Rao, C.C. et al. Unique assemblies of alternating positively and negatively charged layers, directed by hydrogen bonds, ionic interactions, and π-stacking in the crystal structures of complexes between mellitic acid (benzenehexacarboxylic acid) and five planar aromatic bases. Journal of Chemical Crystallography 33, 727–749 (2003). https://doi.org/10.1023/A:1026151222486
Issue Date:
DOI: https://doi.org/10.1023/A:1026151222486