Abstract
Purpose. The objectives of this study were to characterize sepia, synthetic, and bovine melanin and to determine their binding characteristics to the drug memantine.
Methods. Physical methods were used to characterize sepia, synthetic, and bovine melanin. Their binding properties toward memantine were determined in deionized water and phosphate-buffered saline (PBS) at 37°C. Melanin-memantine binding was measured indirectly by determining the unbound fraction of memantine. Curve fitting according to the Langmuir binding isotherm for one binding site was used for the determination of binding capacity (B Lmax ) and dissociation constant (K D ).
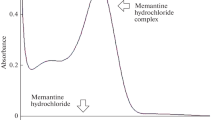
Results. Synthetic and sepia melanin had comparable Gaussian particle size distributions, whereas bovine melanin showed a heterogeneous distribution profile. The suspension medium had a small effect on the particle size distribution of synthetic and bovine melanin. There were characteristic differences in the infrared spectra of the melanins. The rank order for B Lmax in deionized water was sepia > bovine > synthetic melanin. However, when the melanins were suspended in PBS, the B Lmax values were lower, and the rank order was bovine > sepia > synthetic. Whereas the K D values for sepia and synthetic melanin remained largely the same in deionized water and PBS, the K D value for bovine melanin in PBS was more than twice than in deionized water.
Conclusions. This study showed that the physical characteristics of the melanins investigated differ markedly. The binding of memantine to melanin is thought to be determined by the different chemistries of the melanins, particle size, and buffer electrolytes.
Similar content being viewed by others
References
R. M. J. Ings. The melanin binding of drugs and its implications. Drug Metab. Rev. 15:1183-1212 (1984).
G. Prota. Melanins and Melanogenesis. Academic Press, London, 1992.
S. E. Forest and J. D. Simon. Wavelength-dependent photoacoustic calorimetry study of melanin. Photochem. Photobiol. 68:296-298 (1998).
U. Schraermeyer, S. Peters, G. Thumann, N. Kociok, and K. Heimann. Melanin granules of retinal pigment epithelium are connected with the lysosomal degradation pathway. Exp. Eye Res. 68:237-245 (1999).
U. Schraermeyer and K. Heimann. Current understanding on the role of retinal pigment epithelium and its pigmentation. Pigm. Cell Res. 12:219-236 (1999).
T. Sarna. Properties and function of the ocular melanin—a photobiophysical view. J. Photoch. Photobio. B. 12:215-258 (1992).
C. M. R. Clancy and J. D. Simon. Ultrastructural organization of eumelanin from Sepia officinalis measured by atomic force microscopy. Biochemistry-US 40:13353-13360 (2001).
J. B. Nofsinger, S. E. Forest, L. M. Eibest, K. A. Gold, and J. D. Simon. Probing the building blocks of eumelanins using scanning electron microscopy. Pigm. Cell Res. 13:179-184 (2000).
A. Pezzella, M. D'Ischia, A. Napolitano, A. Palumbo, and G. Prota. An integrated approach to the structure of sepia melanin. evidence for a high proportion of degraded 5,6-dihydroxyindole-2-carboxylic acid units in the pigment backbone. Tetrahedron 53:8281-8286 (1997).
L. Novellino, A. Napolitano, and G. Prota. Isolation and characterization of mammalian eumelanins from hair and irides. Biochim. Biophys. Acta 1475:295-306 (2000).
M. Olivieri and R. A. Nicolaus. On the structure of DHI-melanin, Rend. Acc. Sci. Fis. Mat. Vol. LXVI, (1999), http://www.tightrope.it/nicolaus/11b.htm.
M. M. Salazar-Bookaman, I. W. Wainer, and P. N. Patil. Relevance of drug-melanin interactions to ocular pharmacology and toxicology. J. Ocul. Pharmacol. 10:217-239 (1994).
J. M. Gallas, G. W. Zajac, T. Sarna, and P. L. Stotter. Structural differences in unbleached and mildly-bleached synthetic tyrosine-derived melanins identified by scanning probe microscopies. Pigm. Cell Res. 13:99-108 (2000).
G. W. Zajac, J. M. Gallas, J. Cheng, M. Eisner, S. C. Moss, and A. E. Alvarado-Swaisgood. The fundamental unit of synthetic melanin: a verification by tunneling microscopy of X-ray scattering results. Biochim. Biophys. Acta 1199:271-278 (1994).
C. M. R. Clancy, J. B. Nofsinger, R. K. Hanks, and J. D. Simon. A hierarchical self-assembly of eumelanin. J. Phys. Chem. B 104:7871-7873 (2000).
R. D. Schoenwald, V. Tandon, D. E. Wurster, and C. F. Barfknecht. Significance of melanin binding and metabolism in the activity of 5-acetoxyacetylimino-4-methyl-delta(2)-1,3,4,-thiadiazoline-2-sulfonamide. Eur. J. Pharm. Biopharm. 46:39-50 (1998).
S. Kristensen, A. L. Orsteen, S. A. Sande, and H. H. Tonnesen. Photoreactivity of biologically-active compounds VII. interaction of antimalarial-drugs with melanin in-vitro as part of phototoxicity screening. J. Photochem. Photobiol. B. 26:87-95 (1994).
S. Persad, J. D. Wiltshire, I. A. Menon, P. K. Basu, and F. Carre. Role of melanin in drug accumulation in the eye and ocular toxic reaction. J. Invest. Dermatol. 87:432(1986).
B. Leblanc, S. Jezequel, T. Davies, G. Hanton, and C. Taradach. Binding of drugs to eye melanin is not predictive of ocular toxicity. Regul. Toxicol. Pharmacol. 28:124-132 (1998).
A. M. Potts. The reaction of uveal pigment in vitro with polycyclic compounds. Invest. Ophth. Visual 3:405-416 (1964).
P. R. Raghavan, P. A. Zane, and S. L. Tripp. Calculation of drug-melanin binding energy using molecular modeling. Experientia 46:77-80 (1990).
A. H. Lowrey, G. R. Famini, V. P. Loumbev, L. Y. Wilson, and J. M. Tosk. Modeling drug-melanin interaction with theoretical linear solvation energy relationships. Pigm. Cell Res. 10:251-256 (1997).
B. S. Larsson. Interaction between chemicals and melanin. Pigm. Cell Res. 6:127-133 (1993).
I. A. Menon, G. E. Trope, P. K. Basu, D. C. Wakeham, and S. D. Persad. Binding of timolol to iris-ciliary body and melanin: an in vitro model for assessing the kinetics and efficacy of long-acting antiglaucoma drugs. J. Ocul. Pharmacol. 5:313-324 (1989).
B. Larsson and H. Tjälve. Studies on the mechanism of drug-binding to melanin. Biochem. Pharmacol. 28:1181-1187 (1978).
H. S. V. Chen, J. W. Pellegrini, S. K. Aggarwal, S. Z. Lei, S. Warach, F. E. Jensen, and S. A. Lipton. Open-channel block of N-methyl-D-aspartate (NMDA) responses by memantine—therapeutic advantage against NMDA receptor-mediated neurotoxicity. J. Neurosci. 12:4427-4436 (1992).
W. A. Lagreze, R. Knorle, M. Bach, and T. J. Feuerstein. Memantine is neuroprotective in a rat model of pressure-induced retinal ischemia. Invest. Ophth. Vis. Sci. 39:1063-1066 (1998).
C. K. Vorwerk, S. A. Lipton, D. Zurakowski, B. T. Hyman, B. A. Sabel, and E. B. Dreyer. Chronic low-dose glutamate is toxic to retinal ganglion cells—toxicity blocked by memantine. Invest. Ophth. Vis. Sci. 37:1618-1624 (1996).
M. J. Koeberle, P. M. Hughes, C. G. Wilson, and G. G. Skellern. Development of a liquid chromatography–mass spectrometric method for measuring the binding of memantine to different melanins. J. Chromatogr. B 787:313-322 (2003).
K. B. Stepien, J. P. Dworzanski, B. Bilinska, M. Porebska-Budny, A. M. Hollek, and T. Wilczok. Catecholamine Melanins. Structural changes induced by copper ions. Biochim. Biophys. Acta 997:49-54 (1989).
L. Bardani, M. G. Bridelli, M. Carbucichio, and P. R. Crippa. Comparative Mössbauer and infrared analysis of iron-containing melanins. Biochim. Biophys. Acta 716:8-15 (1982).
R. A. Nicolaus and M. Piattelli. Structure of melanins and melanogenesis. J. Polym. Sci. 58:1133-1139 (1962).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Koeberle, M.J., Hughes, P.M., Skellern, G.G. et al. Binding of Memantine to Melanin: Influence of Type of Melanin and Characteristics. Pharm Res 20, 1702–1709 (2003). https://doi.org/10.1023/A:1026116208008
Issue Date:
DOI: https://doi.org/10.1023/A:1026116208008