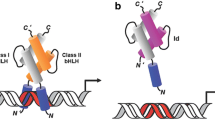
Abstract
The basic helix-loop-helix (bHLH) family of transcription factors functions in the coordinated regulation of gene expression, cell lineage commitment, and cell differentiation in most mammalian tissues. Helix-loop-helix Id (Inhibitor of DNA binding) proteins are distinct from bHLH transcription factors in that they lack the basic domain necessary for DNA binding. Id proteins thus function as dominant negative regulators of bHLH transcription factors. The inhibition of bHLH factor activity by forced constitutive expression of Id proteins is closely associated with the inhibition of differentiation in a number of different cell types, including mammary epithelial cells. Moreover, recent literature suggests important roles of HLH proteins in many normal and transformed tissues, including mammary gland. Therefore, future directions for prognosis or therapeutic treatments of breast cancer may be able to exploit bHLH and Id genes as useful molecular targets. The purpose of this review is to summarize the evidence implicating HLH proteins in the regulation of normal and transformed mammary epithelial cell phenotypes.
Similar content being viewed by others
REFERENCES
C. Murre, P. S. McCaw, and D. Baltimore (1989). A new DNA binding and dimerization motif in immunoglobulin enhancer binding, daughterless, MyoD, and myc proteins. Cell 56(5):777–783.
M. E. Massari and C. Murre (2000). Helix-loop-helix proteins: Regulators of transcription in eucaryotic organisms. Mol. Cell. Biol. 20(2):429–440.
S. Campuzano (2001). Emc, a negative HLH regulator with multiple functions in Drosophila development. Oncogene 20(58):8299–8307.
Y. Yokota (2001). Id and development. Oncogene 20(58):8290–8298.
W. R. Atchley and W. M. Fitch (1997). A natural classification of the basic helix-loop-helix class of transcription factors. Proc. Natl. Acad. Sci. U.S.A. 94(10):5172–5176.
I. Engel and C. Murre (2001). The function of E-and Id proteins in lymphocyte development. Nat. Rev. Immunol. 1(3):193–199.
J. D. Norton, R. W. Deed, G. Craggs, and F. Sablitzky (1998). Id helix-loop-helix proteins in cell growth and differentiation. Trends. Cell. Biol. 8(2):58–65.
R. Benezra (2001). The Id proteins: Targets for inhibiting tumor cells and their blood supply. Biochim. Biophys. Acta. 1551(2):F39-F47.
D. Lyden, A. Z. Young, D. Zagzag, W. Yan, W. Gerald, R. O'Reilly, B. L. Bader, R. O. Hynes, Y. Zhuang, K. Manova, R. Benezra (1999). Id1 and Id3 are required for neurogenesis, angiogenesis and vascularization of tumour xenografts. Nature 401(6754):670–677.
Y. Jen, K. Manova, and R. Benezra (1996). Expression patterns of Id1, Id2, and Id3 are highly related but distinct from that of Id4 during mouse embryogenesis. Dev Dyn. 207(3):235–252.
J. D. Norton (2000). ID helix-loop-helix proteins in cell growth, differentiation and tumorigenesis. J. Cell. Sci. 113(Pt. 22):3897–3905.
R. Rivera and C. Murre (2001). The regulation and function of the Id proteins in lymphocyte development. Oncogene 20(58):8308–8316.
Y. Yokota and S. Mori (2002). Role of Id family proteins in growth control. J. Cell. Physiol. 190(1):21–28.
R. Benezra, S. Rafii, and D. Lyden (2001). The Id proteins and angiogenesis. Oncogene 20(58):8334–8341.
Z. Zebedee and E. Hara (2001). Id proteins in cell cycle control and cellular senescence. Oncogene 20(58):8317–8325.
P. J. Andres-Barquin, M. C. Hernandez, and M. A. Israel (2000). Id genes in nervous system development. Histol. Histopathol. 15(2):603–618.
C. Murre, G. Bain, M. A. van Dijk, I. Engel, B. A. Furnari, M. E. Massari, J. R. Matthews, M. W. Quong, R. R. Rivera, and M. H. Stuiver (1994). Structure and function of helix-loop-helix proteins. Biochim. Biophys. Acta 1218(2):129–135.
A. Ephrussi, G. M. Church, S. Tonegawa, W. Gilbert (1985). B lineage—Specific interactions of an immunoglobulin enhancer with cellular factors in vivo. Science 227(4683):134–140.
R. Benezra, R. L. Davis, D. Lockshon, D. L. Turner, and H. Weintraub (1990). The protein Id: A negative regulator of helix-loop-helix DNA binding proteins. Cell 61(1):49–59.
H. M. Ellis, D. R. Spann, and J. W. Posakony (1990). Extramacrochaetae, a negative regulator of sensory organ development in Drosophila, defines a new class of helix-loop-helix proteins. Cell 61(1):27–38.
A. Lasorella, T. Uo, and A. Iavarone (2001). Id proteins at the cross-road of development and cancer. Oncogene 20(58):8326–8333.
J. P. Coppe, A. P. Smith, and P. Y. Desprez (2003). Id proteins in epithelial cells. Exp. Cell. Res. 285(1):131–145.
L. Hennighausen and G. W. Robinson (1998). Think globally, act locally: The making of a mouse mammary gland. Genes. Dev. 12(4):449–455.
R. Strange, T. Metcalfe, L. Thackray, and M. Dang (2001). Apoptosis in normal and neoplastic mammary gland development. Microsc. Res. Tech. 52(2):171–181.
V. Djonov, A. C. Andres, and A. Ziemiecki (2001). Vascular remodelling during the normal and malignant life cycle of the mammary gland. Microsc. Res. Tech. 52(2):182–189.
J. Russo, Y. F. Hu, I. D. Silva, and I. H. Russo (2001). Cancer risk related to mammary gland structure and development. Microsc. Res. Tech. 52(2):204–223.
A. Clamp, S. Danson, and M. Clemons (2002). Hormonal risk factors for breast cancer: Identification, chemoprevention, and other intervention strategies. Lancet. Oncol. 3(10):611–619.
P. Henthorn, M. Kiledjian, and T. Kadesch (1990). Two distinct transcription factors that bind the immunoglobulin enhancer microE5/kappa 2 motif. Science 247(4941):467–470.
P. Dias, M. Dilling, and P. Houghton (1994). The molecular basis of skeletal muscle differentiation. Semin. Diagn. Pathol. 11(1):3–14.
S. Desiderio (1995). Lymphopoiesis. Transcription factors controlling B-cell development. Curr. Biol. 5(6):605–608.
M. H. Farah, J. M. Olson, H. B. Sucic, R. I. Hume, S. J. Tapscott, and D. L. Turner (2000). Generation of neurons by transient expression of neural bHLH proteins in mammalian cells. Development 127(4):693–702.
J. Chaudhary, A. S. Cupp, and M. K. Skinner (1997). Role of basic-helix-loop-helix transcription factors in Sertoli cell differentiation: Identification of an E-box response element in the transferrin promoter. Endocrinology. 138(2):667–675.
S. T. Park, G. P. Nolan, and X. H. Sun (1999). Growth inhibition and apoptosis due to restoration of E2A activity in T cell acute lymphoblastic leukemia cells. J. Exp. Med. 189(3):501–508.
H. Weintraub, R. Davis, S. Tapscott, M. Thayer, M. Krause, R. Benezra, T. K. Blackwell, D. Turner, R. Rupp, S. Hollenberg, Y. Zhuang and A. Lassar. (1991) The myoD gene family: Nodal point during specification of the muscle cell lineage. Science 251(4995):761–766.
E. N. Olson (1990). MyoD family: A paradigm for development? Genes Dev. 4(9):1454–1461.
R. Kageyama, M. Ishibashi, K. Takebayashi, and K. Tomita (1997). bHLH transcription factors and mammalian neuronal differentiation. Int. J. Biochem. Cell. Biol. 29(12):1389–1399.
F. J. Naya, H. P. Huang, Y. Qiu, H. Mutoh, F. J. DeMayo, A. B. Leiter, and M. J. Tsai (1997). Diabetes, defective pancreatic morphogenesis, and abnormal enteroendocrine differentiation in BETA2/neuroD-deficient mice. Genes Dev. 11(18):2323–2334.
D. Srivastava (1999). HAND proteins: Molecular mediators of cardiac development and congenital heart disease. Trends Cardiovasc Med. 9(1/2):11–18.
R. Kageyama, Y. Sasai, C. Akazawa, M. Ishibashi, K. Takebayashi, C. Shimizu, K. Tomita, and S. Nakanishi (1995). Regulation of mammalian neural development by helix-loop-helix transcription factors. Crit. Rev. Neurobiol. 9(2/3):177–188.
D. B. Spicer, J. Rhee, W. L. Cheung, and A. B. Lassar (1996). Inhibition of myogenic bHLH and MEF2 transcription factors by the bHLH protein Twist. Science. 272(5267):1476–1480.
C. Lemercier, R. Q. To, R. A. Carrasco, and S. F. Konieczny (1998). The basic helix-loop-helix transcription factor Mist1 functions as a transcriptional repressor of myoD. EMBO J. 17(5):1412–1422.
C. L. Pin, A. C. Bonvissuto, and S. F. Konieczny (2000). Mist1 expression is a common link among serous exocrine cells exhibiting regulated exocytosis. Anat. Rec. 259(2):157–167.
R. A. Shivdasani, E. L. Mayer, and S. H. Orkin (1995). Absence of blood formation in mice lacking the T-cell leukaemia oncoprotein tal-1/SCL. Nature. 373(6513):432–434.
I. Vastrik, T. P. Makela, P. J. Koskinen, J. Klefstrom, and K. Alitalo (1994). Myc protein: Partners and antagonists. Crit. Rev. Oncog. 5(1):59–68.
F. Javaux, A. Donda, G. Vassart, and D. Christophe (1991). Cloning and sequence analysis of TFE, a helix-loop-helix transcription factor able to recognize the thyroglobulin gene promoter in vitro. Nucleic. Acids. Res. 19(5):1121–1127.
M. S. Brown and J. L. Goldstein (1997). The SREBP pathway: Regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 89(3):331–340.
P. J. Hurlin, D. E. Ayer, C. Grandori, and R. N. Eisenman (1994). The Max transcription factor network: Involvement of Mad in differentiation and an approach to identification of target genes. Cold Spring Harb. Symp. Quant. Biol. 59:109–116.
P. Muller, S. Kietz, J. A. Gustafsson, and A. Strom (2002). The anti-estrogenic effect of all-trans-retinoic acid on the breast cancer cell line MCF-7 is dependent on HES-1 expression. J. Biol. Chem. 277(32):28376–28379.
A. M. Henderson, S. J. Wang, A. C. Taylor, M. Aitkenhead, and C. C. Hughes (2001). The basic helix-loop-helix transcription factor HESR1 regulates endothelial cell tube formation. J. Biol. Chem. 276(9):6169–6176.
C. Qin, C. Wilson, C. Blancher, M. Taylor, S. Safe, and A. L. Harris (2001). Association of ARNT splice variants with estrogen receptor-negative breast cancer, poor induction of vascular endothelial growth factor under hypoxia, and poor prognosis. Clin. Cancer. Res. 7(4):818–823.
O. Hankinson (1995). The aryl hydrocarbon receptor complex. Annu. Rev. Pharmacol. Toxicol. 35:307–340.
H. I. Swanson and C. A. Bradfield (1993). The AH-receptor: Genetics, structure and function. Pharmacogenetics 3(5):213–230.
G. Semenza (2002). Signal transduction to hypoxia-inducible factor 1. Biochem. Pharmacol. 64(5/6):993–998.
R. W. Deed, M. Jasiok, and J. D. Norton (1994). Nucleotide sequence of the cDNA encoding human helix-loop-helix Id-1 protein: Identification of functionally conserved residues common to Id proteins. Biochim. Biophys. Acta. 1219(1):160–162.
X. H. Sun, N. G. Copeland, N. A. Jenkins, and D. Baltimore (1991). Id proteins Id1 and Id2 selectively inhibit DNA binding by one class of helix-loop-helix proteins. Mol. Cell. Biol. 11(11):5603–5611.
J. Biggs, E. V. Murphy, and M. A. Israel (1992). A human Id-like helix-loop-helix protein expressed during early development. Proc. Natl. Acad. Sci. U.S.A. 89(4):1512–1516.
B. A. Christy, L. K. Sanders, L. F. Lau, N. G. Copeland, N. A. Jenkins, and D. Nathans (1991). An Id-related helix-loop-helix protein encoded by a growth factor-inducible gene. Proc. Natl. Acad. Sci. U.S.A. 88(5):1815–1819.
W. Ellmeier, A. Aguzzi, E. Kleiner, R. Kurzbauer, and A. Weith (1992). Mutually exclusive expression of a helix-loop-helix gene and N-myc in human neuroblastomas and in normal development. EMBO J. 11(7):2563–2571.
V. Riechmann, I. van Cruchten and F. Sablitzky (1994). The expression pattern of Id4, a novel dominant negative helix-loop-helix protein, is distinct from Id1, Id2 and Id3. Nucleic. Acids. Res. 22(5):749–755.
A. Pagliuca, P. C. Bartoli, S. Saccone, G. Della Valle, and L. Lania (1995). Molecular cloning of ID4, a novel dominant negative helix-loop-helix human gene on chromosome 6p21.3-p22. Genomics 27(1):200–203.
R. Wilson and T. Mohun (1995). XIdx, a dominant negative regulator of bHLH function in early Xenopus embryos. Mech. Dev. 49(3):211–222.
S. Sawai and J. A. Campos-Ortega (1997). A zebrafish Id homologue and its pattern of expression during embryogenesis. Mech. Dev. 65(1/2):175–185.
M. Shirakata, F. K. Friedman, Q. Wei, and B. M. Paterson (1993). Dimerization specificity of myogenic helix-loop-helix DNA-binding factors directed by nonconserved hydrophilic residues. Genes Dev. 7(12A):2456–2470.
A. N. Goldfarb, K. Lewandowska, and C. A. Pennell (1998). Identification of a highly conserved module in E proteins required for in vivo helix-loop-helix dimerization. J. Biol. Chem. 273(5):2866–2873.
P. C. Ma, M. A. Rould, H. Weintraub, and C. O. Pabo (1994). Crystal structure of MyoD bHLH domain-DNA complex: Perspectives on DNA recognition and implications for transcriptional activation. Cell. 77(3):451–459.
T. Ellenberger, D. Fass, M. Arnaud, and S. C. Harrison (1994). Crystal structure of transcription factor E47: E-box recognition by a basic region helix-loop-helix dimer. Genes Dev. 8(8):970–980.
S. Pesce and R. Benezra (1993). The loop region of the helix-loop-helix protein Id1 is critical for its dominant negative activity. Mol. Cell. Biol. 13(12):7874–7880.
J. Wibley, R. Deed, M. Jasiok, K. Douglas, and J. Norton (1996). A homology model of the Id-3 helix-loop-helix domain as a basis for structure–function predictions. Biochim. Biophys. Acta. 1294(2):138–146.
E. Hara, M. Hall, and G. Peters (1997). Cdk2-dependent phosphorylation of Id2 modulates activity of E2A-related transcription factors. EMBO J. 16(2):332–342.
R. W. Deed, E. Hara, G. T. Atherton, G. Peters, and J. D. Norton (1997). Regulation of Id3 cell cycle function by Cdk-2-dependent phosphorylation. Mol. Cell. Biol. 17(12):6815–6821.
R. W. Deed, S. Armitage, M. Brown, and J. D. Norton (1996). Regulation of Id-HLH transcription factor function in third messenger signalling. Biochem. Soc. Trans. 24(1):5S.
M. A. Bounpheng, J. J. Dimas, S. G. Dodds, and B. A. Christy (1999). Degradation of Id proteins by the ubiquitin-proteasome pathway. FASEB J. 13(15):2257–2264.
G. Anand, X. Yin, A. K. Shahidi, L. Grove, and E. V. Prochownik (1997). Novel regulation of the helix-loop-helix protein Id1 by S5a, a subunit of the 26 S proteasome. J. Biol. Chem. 272(31):19140–19151.
R. W. Deed, S. Armitage, and J. D. Norton (1996). Nuclear localization and regulation of Id protein through an E protein-mediated chaperone mechanism. J. Biol. Chem. 271(39):23603–23606.
E. Hara, T. Yamaguchi, H. Nojima, T. Ide, J. Campisi, H. Okayama, and K. Oda (1994). Id-related genes encoding helix-loop-helix proteins are required for G1 progression and are repressed in senescent human fibroblasts. J. Biol. Chem. 269(3):2139–2145.
J. O. Nehlin, E. Hara, W. L. Kuo, C. Collins, J. Campisi (1997). Genomic organization, sequence, and chromosomal localization of the human helix-loop-helix Id1 gene. Biochem. Biophys. Res. Commun. 231(3):628–634.
R. W. Deed, T. Hirose, E. L. Mitchell, M. F. Santibanez-Koref, and J. D. Norton (1994). Structural organisation and chromosomal mapping of the human Id-3 gene. Gene. 151(1/2):309–314.
R. W. Deed, M. Jasiok, and J. D. Norton (1996). Attenuated function of a variant form of the helix-loop-helix protein, Id-3, generated by an alternative splicing mechanism. FEBS Lett. 393(1):113–116.
M. Kurabayashi, R. Jeyaseelan, and L. Kedes (1993). Two distinct cDNA sequences encoding the human helix-loop-helix protein Id2. Gene. 133(2):305–306.
M. V. Barone, R. Pepperkok, F. A. Peverali, and L. Philipson (1994). Id proteins control growth induction in mammalian cells. Proc. Natl. Acad. Sci. U.S.A. 91(11):4985–4988.
P. H. King, T. D. Levine, R. T. FremeauJr., and J. D. Keene (1994). Mammalian homologs of Drosophila ELAV localized to a neuronal subset can bind in vitro to the 3′ UTR of mRNA encoding the Id transcriptional repressor. J. Neurosci. 14(4):1943–1952.
J. D. Keene (2001). Ribonucleoprotein infrastructure regulating the flow of genetic information between the genome and the proteome. Proc. Natl. Acad. Sci. U.S.A. 98(13):7018–7024.
J. Singh, Y. Itahana, S. Parrinello, K. Murata, and P. Y. Desprez (2001). Molecular cloning and characterization of a zinc finger protein involved in Id-1-stimulated mammary epithelial cell growth. J. Biol. Chem. 276(15):11852–11858.
S. Parrinello, C. Q. Lin, K. Murata, Y. Itahana, J. Singh, A. Krtolica, J. Campisi, and P. Y. Desprez (2001). Id-1, ITF-2, and Id-2 comprise a network of helix-loop-helix proteins that regulate mammary epithelial cell proliferation, differentiation, and apoptosis. J. Biol. Chem. 276(42):39213–39219.
P. Y. Desprez, C. Q. Lin, N. Thomasset, C. J. Sympson, M. J. Bissell, and J. Campisi (1998). A novel pathway for mammary epithelial cell invasion induced by the helix-loop-helix protein Id-1. Mol. Cell. Biol. 18(8):4577–4588.
P. Y. Desprez, E. Hara, M. J. Bissell, and J. Campisi (1995). Suppression of mammary epithelial cell differentiation by the helix-loop-helix protein Id-1. Mol. Cell. Biol. 15(6):3398–3404.
P. L. Woo, A. Cercek, P. Y. Desprez, and G. L. Firestone (2000). Involvement of the helix-loop-helix protein Id-1 in the glucocorticoid regulation of tight junctions in mammary epithelial cells. J. Biol. Chem. 275(37):28649–28658.
K. Miyoshi, B. Meyer, P. Gruss, Y. Cui, J. P. Renou, F. V. Morgan, G. H. Smith, M. Reichenstein, M. Shani, L. Hennighausen, and G. W. Robinson (2002). Mammary epithelial cells are not able to undergo pregnancy-dependent differentiation in the absence of the helix-loop-helix inhibitor Id2. Mol. Endocrinol. 16(12):2892–2901.
S. Mori, S. I. Nishikawa, and Y. Yokota (2000). Lactation defect in mice lacking the helix-loop-helix inhibitor Id2. EMBO J. 19(21):5772–5781.
C. Q. Lin, J. Singh, K. Murata, Y. Itahana, S. Parrinello, S. H. Liang, C. E. Gillett, J. Campisi, and P. Y. Desprez (2000). A role for Id-1 in the aggressive phenotype and steroid hormone response of human breast cancer cells. Cancer. Res. 60(5):1332–1340.
J. Singh, K. Murata, Y. Itahana, and P. Y. Desprez (2002). Constitutive expression of the Id-1 promoter in human metastatic breast cancer cells is linked with the loss of NF-1/Rb/HDAC-1 transcription repressor complex. Oncogene 21(12):1812–1822.
S. F. Schoppmann, M. Schindl, G. Bayer, K. Aumayr, J. Dienes, R. Horvat, M. Rudas, M. Gnant, R. Jakesz, and P. Birner (2003). Overexpression of Id-1 is associated with poor clinical outcome in node negative breast cancer. Int. J. Cancer. 104(6):677–682.
J. H. Clement, N. Marr, A. Meissner, M. Schwalbe, W. Sebald, K. O. Kliche, K. Hoffken, S. Wolfl (2000). Bone morphogenetic protein 2 (BMP-2) induces sequential changes of Id gene expression in the breast cancer cell line MCF-7. J. Cancer Res. Clin. Oncol. 126(5):271–279.
S. Fong, Y. Itahana, T. Sumida, J. Singh, J. P. Coppe, Y. Liu, P. C. Richards, J. L. Bennington, N. M. Lee, R. J. Debs, and P. Y. Desprez (manuscript submitted for publication) Id-1 is a new molecular target in breast cancer cell invasion and metastasis.
Y. Itahana, J. Singh, T. Sumida, J. P. Coppe, J. L. Bennington, and P. Y. Desprez (manuscript submitted for publication). Role of Id-2 in the maintenance of a differentiated and non-invasive phenotype in breast cancer cells.
C. Beger, L. N. Pierce, M. Kruger, E. G. Marcusson, J. M. Robbins, P. Welcsh, P. J. Welch, K. Welte, M. C. King, J. R. Barber, and F. Wong-Staal (2001). Identification of Id4 as a regulator of BRCA1 expression by using a ribozyme-library-based inverse genomics approach. Proc. Natl. Acad. Sci. U.S.A. 98(1):130–135.
L. Hennighausen and G. W. Robinson (2001). Signaling pathways in mammary gland development. Dev. Cell. 1(4):467–475.
C. Schmidhauser, M. J. Bissell, C. A. Myers, and G. F. Casperson (1990). Extracellular matrix and hormones transcriptionally regulate bovine beta-casein 5′ sequences in stably transfected mouse mammary cells. Proc. Natl. Acad. Sci. U.S.A. 87(23):9118–9122.
P. Y. Desprez, C. Roskelley, J. Campisi, and M. J. Bissell (1993). Isolation of functional cell lines from a mouse mammary cell strain: The importance of basement membrane and cell-cell interaction. Mol. Cell. Differ. 1:99–110.
C. D. Roskelley, P. Y. Desprez, and M. J. Bissell (1994). Extracellular matrix-dependent tissue-specific gene expression in mammary epithelial cells requires both physical and biochemical signal transduction. Proc. Natl. Acad. Sci. U.S.A. 91(26):12378–12382.
N. Uehara, Y. C. Chou, J. J. Galvez, P. de-Candia, R. D. Cardiff, R. Benezra, and G. Shyamala (2003). Id-1 is not expressed in the luminal epithelial cells of mammary glands, Breast. Cancer Res. 5:R25-R29.
I. S. Skerjanc, J. Truong, P. Filion, and M. W. McBurney (1996). A splice variant of the ITF-2 transcript encodes a transcription factor that inhibits MyoD activity. J. Biol. Chem. 271(7):3555–3561.
K. Neuman, H. O. Nornes, and T. Neuman (1995). Helix-loop-helix transcription factors regulate Id2 gene promoter activity. FEBS Lett. 374(2):279–283.
A. Swarbrick, L. J. Hunter, C. S. Lee, C. M. Sergio, R. L. Sutherlan, and E. A. Musgrove Id1 is a critical target of c-myc in breast cancer cells. Molecular Biology of the Cell, 42nd American Society for Cell Biology Annual Meeting, Abstract 2438.
R. J. Akhurst and R. Derynck (2001). TGF-beta signaling in cancer—A double-edged sword. Trends Cell Biol. 11(11):S44-S51.
T. Ogata, J. M. Wozney, R. Benezra, and M. Noda (1993). Bone morphogenetic protein 2 transiently enhances expression of a gene, Id (inhibitor of differentiation), encoding a helix-loop-helix molecule in osteoblast-like cells. Proc. Natl. Acad. Sci. U.S.A. 90(19):9219–9222.
T. Katagiri, A. Yamaguchi, M. Komaki, E. Abe, N. Takahashi, T. Ikeda, V. Rosen, J. M. Wozney, A. Fujisawa-Sehara, and T. Suda (1994). Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J. Cell. Biol. 127(6, Pt. 1):1755–1766.
O. Korchynskyi and P. ten Dijke (2002). Identification and functional characterization of distinct critically important bone morphogenetic protein-specific response elements in the Id1 promoter. J. Biol. Chem. 277(7):4883–4891.
T. Lopez-Rovira, E. Chalaux, J. Massague, J. L. Rosa, and F. Ventura (2002). Direct binding of Smad1 and Smad4 to two distinct motifs mediates bone morphogenetic protein-specific transcriptional activation of Id1 gene. J. Biol. Chem. 277(5):3176–3185.
P. L. Davis, A. Miron, L. M. Andersen, J. D. Iglehart, and J. R. Marks (1999). Isolation and initial characterization of the BRCA2 promoter. Oncogene 18(44):6000–6012.
F. T. Kolligs, M. T. Nieman, I. Winer, G. Hu, D. Van Mater, Y. Feng, I. M. Smith, R. Wu, Y. Zhai, K. R. Cho, and E. R. Fearon (2002). ITF-2, a downstream target of the Wnt/TCF pathway, is activated in human cancers with beta-catenin defects and promotes neoplastic transformation. Cancer Cell 1(2):145–155.
P. Polakis (2000). Wnt signalling and cancer. Genes Dev. 14:1837–1851.
J. S. Michaelson and P. Leder (2001). Beta-catenin is a downstream effector of Wnt-mediated tumorigenesis in the mammary gland. Oncogene 20(37):5093–5099.
R. C. Gallagher, T. Hay, V. Meniel, C. Naughton, T. J. Anderson, H. Shibata, M. Ito, H. Clevers, T. Noda, O. J. Sansom, J. O. Mason, and A. R. Clarke (2002). Inactivation of Apc perturbs mammary development, but only directly results in acanthoma in the context of Tcf-1 deficiency. Oncogene 21(42):6446–6457.
M. Bien and H. Clevers (2000). Linking colorectal cancer to Wnt signaling. Cell 103(2):311–320.
J. P. Thiery (2002). Epithelial-mesenchymal transitions in tumor progression. Nat. Rev. Cancer. 2:442–454.
M. A. Perez-Moreno, A. Locascio, I. Rodrigo, G. Dhondt, F. Portillo, M. A. Nieto, and A. Cano (2001). A new role for E12/E47 in the repression of E-cadherin expression and epithelial-mesenchymal transitions. J. Biol. Chem. 276(29):27424–27431.
M. Schindl, S. F. Schoppmann, T. Strobel, H. Heinzl, C. Leisser, R. Horvat, and P. Birner (2003). Level of Id-1 protein expression correlates with poor differentiation, enhanced malignant potential, and more aggressive clinical behavior of epithelial ovarian tumors. Clin. Cancer. Res. 9(2):779–785.
M. Schindl, G. Oberhuber, A. Obermair, S. F. Schoppmann, B. Karner, and P. Birner (2001). Overexpression of Id-1 protein is a marker for unfavorable prognosis in early-stage cervical cancer. Cancer Res. 61(15):5703–5706.
N. Takai, T Miyazaki, K. Fujisawa, K. Nasu, and I. Miyakawa (2001). Id1 expression is associated with histological grade and invasive behavior in endometrial carcinoma. Cancer Lett. 165(2):185–193.
M. A. Israel, M. C. Hernandez, M. Florio, P. J. Andres-Barquin, A. Mantani, J. H. Carter, and C. M. Julin (1999). Id gene expression as a key mediator of tumor cell biology. Cancer. Res. 59( 7 Suppl.):1726s-1730s.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Desprez, PY., Sumida, T. & Coppé, JP. Helix-Loop-Helix Proteins in Mammary Gland Development and Breast Cancer. J Mammary Gland Biol Neoplasia 8, 225–239 (2003). https://doi.org/10.1023/A:1025957025773
Issue Date:
DOI: https://doi.org/10.1023/A:1025957025773