Abstract
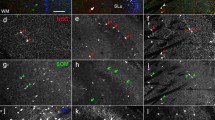
A column of parvalbumin immunoreactive neurons is closely associated with the location of respiratory neurons in the ventrolateral medulla of the rat. The majority (66%) of bulbospinal neurons in the medullary ventral respiratory column (VRC) that were retrogradely labeled by tracer injections in the phrenic nucleus were also positive for parvalbumin. In contrast, only 18.8% of VRC neurons retrogradely labeled after a tracer injection in the VRC, also expressed parvalbumin. The average cross-sectional area of VRC neurons retrogradely labeled after VRC injections was 193.8 μm2 ± 6.6 SE. These were significantly smaller than VRC parvalbumin neurons (271.9 μm2 ± 12.3 SE). Parvalbumin neurons were found in the Bötzinger Complex, the rostral ventral respiratory group (VRG), and the caudal VRG, areas which all contribute to the bulbospinal projection. In contrast, parvalbumin neurons were sparse or absent in the preBötzinger Complex and in the vicinity of the retrotrapezoid nucleus, areas that have few bulbospinal projections. Parvalbumin was rarely colocalized within Neurokinin-1 receptor positive (NK1R) VRC neurons, which are found in the preBötzinger complex and in the anteroventral part of the rostral VRG. Parvalbumin neurons in the Bötzinger Complex and rostral VRG help define the rostrocaudal extent of these regions. The absence of parvalbumin neurons from the intervening preBötzinger complex also helps establish the boundaries of this region. Regional boundaries described in this manner are in good agreement with earlier physiological and anatomical studies. Taken together, the distributions of parvalbumin, NK1R and bulbospinal neurons suggest that the rostral VRG may be subdivided into distinct, anterodorsal, anteroventral, and posterior subdivisions.
Similar content being viewed by others
References
Alheid, G. F., Hayashi, F., Miller, J. F. & McCrimmon, D. R. (2000) Retrograde labeling of parvalbumin neurons in the ventral medullary respiratory group in the rat. Soc Neurosci Abs #384.4.
Alheid, G. F., Gray, P. A., Habtemarkos, R., Feldman, J. L. & McCrimmon, D. R. (2001) Calcium binding proteins, NK1 receptors, and compartments in the ventral respiratory group of the rat. Soc Neurosci Abs #633.1.
Ballanyi, K., Onimaru, H. & Homma, I. (1999) Respiratory network function in the isolated brainstemspinal cord of newborn rats. Prog Neurobiol 59, 583–634.
Baimbridge, K. G., Celio, M. R. & Rogers, J. H. (1992) Calcium-binding proteins in the nervous-system. Trends in Neurosciences 15, 303–308.
Bianchi, A. L., Denavit-SaubiÉ, M. & Champagnat, J. (1995) Central control of breathing in mammals—Neuronal circuitry, membraneproperties, and neurotransmitters. Physiol Rev 75, 1–45.
Blessing, W. W. (1990) Distribution of glutamic decarboxylase-containing neurons in rabbit medulla oblongata with attention to intramedullary and spinal projections. Neurosci 37, 171–185.
Bryant, T. H., Yoshida, S., de Castro, D. & Lipski, J. (1993) Expiratory neurons of the Bötzinger Complex in the rat: A morphological study following intracellular labeling with biocytin. J Comp Neurol 335, 267–282.
Caillard, O., Moreno, H., Schwaller, B., Llano, I., Celio, M. R. & Marty, A. (2000) Role of the calcium-binding protein parvalbumin in short-term synaptic plasticity. Proc Natl Acad Sci USA 97, 13372–13377.
Celio, M. R. & Heizmann, C. W. (1981) Calciumbinding protein parvalbumin as a neuronal marker. Nature 293, 300–302.
Celio, M. R. (1990) Calbindin D-28k and parvalbumin in the rat nervous system. Neurosci 35, 375–475.
Connelly, C. A., Ellenberger, H. H. & Feldman, J. L. (1990) Respiratory activity in retrotrapezoid nucleus in cat. Am J Physiol 258, L33–L44.
Connelly, C. A., Dobbins, E. G. & Feldman, J. L. (1992) Pre-Bötzinger complex in cats: Respiratory neuronal discharge patterns. Brain Res 590, 337–340.
Cox, M. & Halliday, G. M. (1993) Parvalbumin as an anatomical marker for discrete subregions of the ambiguus complex in the rat. Neurosci Lett 160, 101–105.
Dobbins, E. G. & Feldman, J. L. (1994) Brainstem network controlling descending drive to phrenic motoneurons in rat. J Comp Neurol 347, 64–86.
Ellenberger, H. H. & Feldman, J. L. (1988) Monosynaptic transmission of respiratory drive to phrenic motoneurons from brainstem bulbospinal neurons in rats. J Comp Neurol 269, 47–57.
Ellenberger, H. H. & Feldman, J. L. (1990) Subnuclear organization of the lateral tegmental field of the rat. I: Nucleus ambiguus and ventral respiratory group. J Comp Neurol 294, 202–211.
Ellenberger, H. H., Feldman, J. L. & Zhan, W. Z. (1990) Subnuclear organization of the lateral tegmental field of the rat. II: Catecholamine neurons and ventral respiratory group. J Comp Neurol 294, 212–222.
Ellenberger, H. H. (1999) Distribution of bulbospinal gamma-aminobutyric acid-synthesizing neurons of the ventral respiratory group of the rat. J Comp Neurol 411, 130–144.
Endo, T., Takazawa, K., Kobayashi, S. & Onaya, T. (1986) Immunochemical and immunohistochemical localization of parvalbumin in rat nervous tissues. J Neurochem 46, 892–898.
Ezure, K. (1990) Synaptic connections between medullary respiratory neurons and considerations on the genesis of respiratory rhythm. Progr Neurobiol 35, 429–450.
Ezure, K., Manabe, M. & Yamada, H. (1988) Distribution of medullary respiratory neurons in the rat. Brain Res 455, 262–270.
Feldman, J. L. (1986) Neurophysiology of breathing in mammals. In Handbook of Physiology, The Nervous System, Sect. 1. pp. 463–524.Washington: American Physiological Society.
Feldman, J. L., Mitchell, G. S. & Nattie, E. E. (2003) Breathing: Rhythmicity, plasticity, chemosensitivity. Annual Rev. Neurosci 26, 239–266.
Feldman, J. L. & McCrimmon, D. R. (2003) Neural control of breathing. In Fundamental Neuroscience (edited by Squire, L. R., Bloom, F. E., McConnell, S. K., Roberts, J. L., Spitzer, N. C. & Zigmond, M. J.) pp. 967–990. San Diego: Academic Press/Elsevier Science.
Feldman, J. L., McCrimmon, D. R. & Speck, D. F. (1984) Effect of synchronous activation of medullary inspiratory bulbo-spinal neurones on phrenic nerve discharge in cat. J Physiol 347, 241–254.
Frermann, D., Keller, B. U. & Richter, D. W. (1999) Calcium oscillations in rhythmically active respiratory neurones in the brainstem of the mouse. J Physiol 515, 119–131.
Goodchild, A. K., Llewellyn-Smith, I. J., Sun, Q.-J., Chalmers, J., Cunningham, A. M. & Pilowsky, P. M. (2000) Calbindin-immunoreactive neurons in the reticular formation of the rat brainstem: Catecholamine content and spinal projections. J Comp Neurol 424, 547–562.
Grace, A. A. & Llinas, R. (1985) Morphological artifacts induced in intracellularly stained neurons by dehydration: Circumvention using rapid dimethyl sulfoxide clearing. Neuroscience 16, 461–475.
Gray, P. A., Rekling, J. C., Bocchiaro, C. M. & Feldman, J. L. (1999) Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the preBötzinger complex. Science 286, 1566–1568.
Gray, P. A., Janczewski, W. A., Mellen, N., McCrimmon, D. R. & Feldman, J. L. (2001) Normal breathing requires preBötzinger complex neurokinin-1 receptor-expressing neurons. Nat Neurosci 4, 927–930.
Guyenet, P. G. & Wang, H. (2001) Pre-Bötzinger neurons with preinspiratory discharges “in vivo” express NK1 receptors in the rat. J Neurophysiol 86, 438–446.
Guyenet, P. G., Sevigny, C. P., Weston, M. C. & Stornetta, R. L. (2002) Neurokinin-1 receptorexpressing cells of the ventral respiratory group are functionally heterogeneous and predominantly glutamatergic. J Neurosci 22, 3806–3816.
Hayashi, F., Coles, S. K. & McCrimmon, D. R. (1996) Respiratory neurons mediating the Breuer-Hering reflex prolongation of expiration in rat. J Neurosci 16, 6526–6536.
Itoh, K., Konishi, A., Nomura, S., Mizuno, N., Nakamura, Y. & Sugimoto, T. (1979) Application of coupled oxidation reaction to electron microscopic demonstration of horseradish peroxidase: Cobaltglucose oxidase method. Brain Research 175, 341–346.
Janczewski, W. A., Onimaru, H., Homma, I. & Feldman, J. L. (2002) Opioid-resistant respiratory pathway from the preinspiratory neurones to abdominal muscles: In vivo and in vitro study in the newborn rat. J. Physiol. 545, 1017–1026.
Johnson, S. M., Smith, J. C., Funk, G. D. & Feldman, J. L. (1994) Pacemaker behavior of respiratory neurons in medullary slices from neonatal rat. J Neurophysiol 72, 2598–2608.
Johnson, S. M., Koshiya, N. & Smith, J. C. (2001) Isolation of the kernel for respiratory rhythm generation in a novel preparation: The pre-Bötzinger complex “island”. J Neurophysiol 85, 1772–1776.
Koshiya, N. & Smith, J. C. (1999) Neuronal pacemaker for breathing visualized in vitro. Nature 400, 360–363.
Lips, M. B. & Keller, B. U. (1998) Endogenous calcium buffering in motoneurones of the nucleus hypoglossus from mouse. J Physiol 511, 105–117.
Lips, M. B. & Keller, B. U. (1999) Activity-related calcium dynamics in motoneurons of the nucleus hypoglossus from mouse. J Neurophysiol 82, 2936–2946.
Lipski, J., Zhang, X., Kruszewska, B. & Kanjhan, R. (1994) Morphological study of long axonal projections of ventral medullary inspiratory neurons in the rat. Brain Res 640, 171–184.
Liu, Y. Y., Ju, G. & Wong-Riley, M. T. (2001) Distribution and colocalization of neurotransmitters and receptors in the pre-Bötzinger complex of rats. J Appl Physiol 91, 1387–1395.
Liu, Q. & Wong-Riley, M. T. (2002) Postnatal expression of neurotransmitters, receptors, and cytochrome oxidase in the rat pre-Bötzinger complex. J Appl Physiol 92, 923–934.
Livingston, C. A. & Berger, A. J. (1989) Immunocytochemical localization of GABA in neurons projecting to the ventrolateral nucleus of the solitary tract. Brain Res 494, 143–150.
Makeham, J. M., Goodchild, A. K. & Pilowsky, P. M. (2001) NK1receptor and the ventral medulla of the rat: Bulbospinalandcatecholaminergic neurons. NeuroReport 12, 3663–3667.
McCrimmon, D. R., Feldman, J. L. & Speck, D. F. (1986) Respiratory motoneuronal activity is altered by injections of picomoles of glutamate into cat brain stem. J Neurosci 6, 2384–2392.
McCrimmon, D. R., Ramirez, J. M., Alford, S. & Zuperku, E. J. (2000) Unraveling the mechanism for respiratory rhythm generation. Bioessays 22, 6–9.
Mellen, N. M., Janczewski, W. A., Bocchiaro, C. M. & Feldman, J. L. (2003) Opioid-induced quantal slowing reveals dual networks for respiratory rhythm generation. Neuron 37, 821–826.
Monnier, A., Alheid, G. F. & McCrimmon, D. R. (2003) Defining ventral medullary respiratory compartments with a glutamate receptor agonist in the rat. J Physiol 548, 859–874.
NÚÑez-Abades, P. A., Pasaro, R. & Bianchi, A. L. (1991) Localization of respiratory bulbospinal and propriobulbar neurons in the region of the nucleus ambiguus of the rat. Brain Res 568, 165–172.
NÚÑez-Abades, P. A., Morillo, A. M. & Pasaro, R. (1993) Brainstem connections of the rat ventral respiratory subgroups: Afferent projections. J Auton Nerv Syst 42, 99–118.
Ogilvie, M. D., Gottschalk, A., Anders, K., Richter, D. W. & Pack, A. I. (1992) A network model of respiratory rhythmogenesis. Am J Physiol 263, R962–R975.
Okazaki, M., Takeda, R., Haji, A. & Yamazaki, H. (2001) Glutamic acid decarboxylase-immunoreactivity of bulbar respiratory neurons identified by intracellular recording and labeling in rats. Brain Res 914, 34–47.
Onai, T. & Miura, M. (1986) Projections of supraspinal structures to the phrenic motor nucleus in cats studied by a horseradish peroxidase microinjection method. J Auton Nerv Syst 16, 61–77.
Onai, T., Saji, M. & Miura, M. (1987) Projections of supraspinal structures to the phrenic motor nucleus in rats studied by a horseradish peroxidase microinjection method. J Auton Nerv Syst 21, 233–239.
Onimaru, H. (1995) Studies of the respiratory center using isolated brainstem-spinal cord preparations. Neurosci Res 21, 183–190.
Onimaru, H., Arata, A. & Homma, I. (1987) Localization of respiratory rhythm-generating neurons in the medulla of brainstem-spinal cord preparations from newborn rats. Neurosci Lett 78, 151–155.
Onimaru, H., Arata, A. & Homma, I. (1997) Neuronal mechanisms of respiratory rhythm generation: An approach using in vitro preparation. Jpn. J. Physiol. 47, 385–403.
Onimaru, H. & Homma, I. (2003) A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J Neurosci 23, 1478–1486.
Otake, K., Sasaki, H., Mannen, H. & Ezure, K. (1987) Morphology of expiratory neurons of the Bötzinger complex: An HRP study in the cat. J Comp Neurol 258, 565–579.
Paxinos, G., Kus, L., Ashwell, K. W. S. & Watson, C. (1999a) Chemoarchitectonic Atlas of The Rat Forebrain. San Diego: Academic Press.
Paxinos, G. C. P., Wang, H. & Wang, P. Y. (1999b) Chemoarchitectonic Atlas of The Rat Brainstem. San Diego: Academic Press.
Pearce, R. A., Stornetta, R. L. & Guyenet, P. G. (1989) Retrotrapezoid nucleus in the rat. Neurosci Lett 101, 138–142.
Pierrefiche, O., Champagnat, J. & Richter, D. W. (1995) Calcium-dependent conductances control neurones involved in termination of inspiration in cats. Neurosci Lett 184, 101–104.
Pilowsky, P. M., Jiang, C. & Lipski, J. (1990) An intracellular study of respiratory neurons in the rostral ventrolateral medulla of the rat and their relationship to catecholamine-containing neurons. J Comp Neurol 301, 604–617.
Plogmann, D. & Celio, M. R. (1993) Intracellular concentration of parvalbumin in nerve cells. Brain Res 600, 273–279.
Portillo, F. & Pasaro, R. (1988) Location of bulbospinal neurons and of laryngeal motoneurons within the nucleus ambiguus of the rat and cat by means of retrograde fluorescent labelling. J Anat 159, 11–18.
Ramirez, J. M. & Richter, D. W. (1996) The neuronal mechanisms of respiratory rhythm generation. Curr Opin Neurobiol 6, 817–825.
Reiner, A., Medina, L., Figueredo-Cardenas, G. & Anfinson, S. (1995) Brain-stem motoneuron pools that are selectively resistant in amyotrophic-lateralsclerosis are preferentially enriched in parvalbumin—evidence from monkey brain-stem for a calciummediated mechanism in sporadic ALS. Exp Neurol 131, 239–250.
Rekling, J. C. & Feldman, J. L. (1998) PreBötzinger complex and pacemaker neurons: Hypothesized site and kernel for respiratory rhythm generation. Annu Rev Physiol 60, 385–405.
Richter, D. W., Champagnat, J., Jacquin, T. & Benacka, R. (1993) Calcium currents and calciumdependent potassium currents in mammalian medullary respiratory neurones. J Physiol 470, 23–33.
Rybak, I. A., Paton, J. F. & Schwaber, J. S. (1997a) Modeling neural mechanisms for genesis of respiratory rhythm and pattern. I. Models of respiratory neurons. J Neurophysiol 77, 1994–2006.
Rybak, I. A., Paton, J. F. & Schwaber, J. S. (1997b) Modeling neural mechanisms for genesis of respiratory Parvalbumin in respiratory neurons 717 rhythm and pattern. II. Network models of the central respiratory pattern generator. J Neurophysiol 77, 2007–2026.
Saito, Y., Tanaka, I. & Ezure, K. (2002) Morphology of the decrementing expiratory neurons in the brainstem of the rat. Neurosci Res 44, 141–153.
Schreihofer, A. M., Stornetta, R. L. & Guyenet, P. G. (1999) Evidence for glycinergic respiratory neurons: Bötzinger neurons express mRNA for glycinergic transporter 2. J Comp Neurol 407, 583–597.
SchÖz, A. & Palm, G. (1989) Density of neurons and synapses in the cerebral cortex of the mouse. J Comp Neurol 286, 442–455.
Schwarzacher, S. W., Wilhelm, Z., Anders, K. & Richter, D. W. (1991) The medullary respiratory network in the rat. J Physiol 435, 631–644.
Schwarzacher, S. W., Smith, J. C. & Richter, D. W. (1995) Pre-Bötzinger complex in the cat. J Neurophysiol 73, 1452–1461.
Skoglund, T. S., Pascher, R. & Berthold, C. H. (1996) Aspects of the quantitative analysis of neurons in the cerebral cortex. J Neurosci Meth 70, 201–210.
Smith, J. C., Morrison, D. E., Ellenberger, H. H., Otto, M. R. & Feldman, J. L. (1989) Brainstem projections to the major respiratory neuron populations in the medulla of the cat. J Comp Neurol 281, 69–96.
Smith, J. C., Ellenberger, H. H., Ballanyi, K., Richter, D. W. & Feldman, J. L. (1991) Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science 254, 726–729.
Smith, J. C., Butera, R. J., Koshiya, N., del Negro, C., Wilson, C. G. & Johnson, S. M. (2000) Respiratory rhythm generation in neonatal and adult mammals: The hybrid pacemaker-network model. Respir Physiol 122, 131–147.
Solomon, I. C., Edelman, N. H., & Neubauer, J. A. (1999) Patterns of phrenic motor output evoked by chemical stimulation of neurons located in the pre-Bötzinger complex in vivo. J Neurophysiol 81, 1150–1161.
Solomon, I. C., Halat, T. J., El-Maghrabi, R. & O'Neal, M. H. 3rd (2001) Differential expression of connexin26andconnexin32 in the pre-Bötzinger complex of neonatal and adult rat. J Comp Neurol 440, 12–19.
Sterio, D. C. (1984) The unbiased estimation ofnumberand sizes of arbitrary particles using the dissector. J Microsc 134, 127–136.
Stornetta, R. L., Sevigny, C. P. & Guyenet, P. G. (2003) Inspiratory augmenting bulbospinal neurons express both glutamatergic and enkephalinergic phenotypes. J Comp Neurol 455, 113–124.
Sun, Q.-J., Goodchild, A. K., Chalmers, J. P. & Pilowsky, P. M. (1998) The pre-Bötzinger complex and phase-spanning neurons in the adult rat. Brain Res 809, 204–213.
Sun, Q.-J., Llewellyn-Smith, I. J., Minson, J. B., Arnolda, L. F., Chalmers, J. P. & Pilowsky, P. M. (1996) Thyrotropin-releasing hormone immunoreactive boutons form close appositions with medullary expiratory neurons in the rat. Brain Res 715, 136–144.
Sun, Q.-J., Minson, J. B., Llewellyn-Smith, I. J., Arnolda, L. F., Chalmers, J. P. & Pilowsky, P. M. (1997) Bötzinger neurons project towards bulbospinal neurons in the rostral ventrolateral medulla of the rat. J Comp Neurol 388, 23–31.
Sun, Q.-J., Pilowsky, P. M., Minson, J., Arnolda, L., Chalmers, J. & Llewellyn-Smith, I. J. (1994) Close appositions between tyrosine hydroxylase immunoreactive boutons and respiratory neurons in the rat ventrolateral medulla. J Comp Neurol 340, 1–10.
Takeda, S., Eriksson, L. I., Yamamoto, Y., Joensen, H., Onimaru, H. & Lindahl, S. G. (2001) Opioid action on respiratory neuron activity of the isolated respiratory network in newborn rats. Anesthesiol 95, 740–749.
Wang, H., Stornetta, R. L., Rosin, D. L. & Guyenet, P. G. (2001) Neurokinin-1 receptorimmunoreactive neurons of the ventral respiratory group in the rat. J Comp Neurol 434, 128–146.
Wang, H., Germanson, T. P. & Guyenet, P. G. (2002) Depressor and tachypneic responses to chemical stimulation of the ventral respiratory group are reduced by ablation of neurokinin-1 receptor-expressing neurons. J Neurosci 22, 3755–3764.
Watson, R. E. Jr., Wiegand, S. J., Clough, R. W. & Hoffman, G. E. (1986) Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides 7, 155–159.
Young, W. S. 3rd, Alheid, G. F. & Heimer, L. (1984) The ventral pallidal projection to the mediodorsal thalamus: A study with fluorescent retrograde tracers and immunohistofluorescence. J Neurosci 4, 1626–1638.
Zhan, W. Z., Ellenberger, H. H. & Feldman, J. L. (1989) Monoaminergic and GABAergic terminations in phrenic nucleus of rat identified by immunohistochemical labeling. Neurosci 31, 105–113.
Zhang, F. T., Wu, Z. A., Li, Z. H. & Li, Y. R. (1991) Effect of blocking medial area of nucleus retrofacialis on respiratory rhythm. Respir Physiol 85, 73–81.
Zheng, Y., Barillot, J. C. & Bianchi, A. L. (1991) Patterns of membrane potentials and distributions of the medullary respiratory neurons in the decerebrate rat. Brain Res 546, 261–270.
Zheng, Y., Barillot, J. C. & Bianchi, A. L. (1992) Medullary expiratory neurons in the decerebrate rat: An intracellular study. Brain Res 576, 245–253.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Alheid, G.F., Gray, P.A., Jiang, M.C. et al. Parvalbumin in respiratory neurons of the ventrolateral medulla of the adult rat. J Neurocytol 31, 693–717 (2002). https://doi.org/10.1023/A:1025799830302
Issue Date:
DOI: https://doi.org/10.1023/A:1025799830302