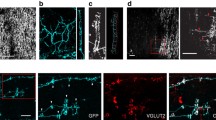
Abstract
Adult rat Purkinje cells are extremely resistant to axotomy and, although they lack spontaneous regeneration, are able to sprout. Axon sprouting is a late process that occurs mainly 6 to 18 months after the lesion and results from an interplay between Purkinje cell intrinsic properties and chemical remodeling of the glial scar. To better appraise the role of the local environment in the late sprouting, we performed new axotomy experiments in mice. In this species, unlike the rat, there is no cavitation because the post-lesional necrotic tissue is invaded by astrocytes and incorporated into the glial scar. In this scarring tissue, chondroitin sulfate proteoglycans (CS-PGs) and PSA-NCAM are present one week after the lesion, but the time courses of their expression differ: the former are transiently expressed and rapidly disappears (by one month), thus preventing early sprouting and providing a negative spatiotemporal correlation with the late sprouting. PSA-NCAM expression, which is maintained up till 12 months, is by itself not sufficient to attract the sprouts, since the core of the glial scar—which exhibits high level of PSA-NCAM—is always devoid of them. Finally, by using a double experimental approach (lesion and graft) aimed at providing a permissive environment to the terminal bulbs of axotomized Purkinje cells, we show that the presence of grafted cerebellum at the lesion site neither changes the time course of the sprouting nor enhances the Purkinje cell axonal regeneration. Nevertheless, these experiments have revealed a new type of altered Purkinje cells, the “irritated” Purkinje cells with a high potentiality for axon sprouting.
Similar content being viewed by others
References
Airaksinen, M. S., Eilers, J., Garaschuk, O., Thoenen, H., Konnerth, A. & Meyer, M. (1997) Ataxia and altered dendritic calcium signaling in mice carrying a targeted null mutation of the calbindin D28k gene. Proc Natl Acad Sci USA 94, 1488–1493.
Alonso, G. & Privat, A. (1993) Reactive astrocytes involved in the formation of lesional scars differ in the mediobasal hypothalamus and in other forebrain regions. J Neurosci Res 34, 523–538.
Amaducci, L., Forno, K. I. & Eng, L. F. (1981) Glial fibrillary acidic protein in cryogenic lesions of the rat brain. Neurosci Lett 21, 27–32.
Armengol, J. A., Sotelo, C., Angaut, P. & Alvarado-Mallart, R. M. (1989) Organization of host afferents to cerebellar grafts implanted into kainate lesioned cerebellum of adult rats. Hodological evidence for the specificity of host-graft interactions. Eur J Neurosci 1, 75–93.
Asher, R. A., Morgenstern, D. A., Moon, L. D. & Fawcett, J. W. (2001) Chondroitin sulphate proteoglycans: Inhibitory components of the glial scar. Prog Brain Res 132, 611–619.
Aubert, I., Ridet, J. L. & Gage, F. H. (1995) Regeneration in the adult mammalian CNS: Guided by development. Curr Opin Neurobiol 5, 625–635.
Austyn, J. M. & Gordon, S. (1981) F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol 11, 805–815.
Avnur, Z. & Geiger, B. (1984) Immunocytochemical localization of native chondroitin-sulfate in tissues and cultured cells using specific monoclonal antibody. Cell 38, 811–822.
Baimbridge, K. G. & Miller, J. J. (1982) Immunohistochemical localization of calcium-binding protein in the cerebellum, hippocampal formation and olfactory bulb of the rat. Brain Res 245, 223–229.
Bignami, A. & Dahl, D. (1976) The astroglial response to stabbing. Immunofluorescence studies with antibodies to astrocyte-specific protein (GFAP) in mammalian and submammalian vertebrates. Neuropathol Appl Neurobiol 2, 99–110.
Bradbury, E. J., Moon, L. D., Popat, R. J., King, V. R., Bennett, G. S., Patel, P. N., Fawcett, J. W. & MCMahon, S. B. (2002) ChondroitinaseABCpromotes functional recovery after spinal cord injury. Nature 416, 636–640.
Bravin, M., Savio, T., Strata, P. & Rossi, F. (1997) Olivocerebellar axon regeneration and target reinnervation following dissociated Schwann cell grafts in surgically injured cerebella of adult rats. Eur J Neurosci 9, 2634–2649.
Bregman, B. S., Kunkel-Bagden, E., Mcatee, M. & O'Neill, A. (1989) Extension of the critical period for developmental plasticity of the corticospinal pathway. J Comp Neurol 282, 355–370.
Cajal, R. S. (1928) Degeneration and Regeneration of the Nervous System. Vol. 1, New York: Hafner.
Caroni, P. (1997) Intrinsic neuronal determinants that promote axonal sprouting and elongation. Bioessays 9, 767–775.
Cremer, H., Lange, R., Christoph, A., Plomann, M., Vopper, G., Roes, J., Brown, R., Baldwin, S., Kraemer, P., Scheff, S., iet al. (1994) Inactivation of the N-CAM gene in mice results in size reduction of the olfactory bulb and deficits in spatial learning. Nature 367, 455–459.
David, S. & Aguayo, A. J. (1981) Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science 214, 931–933.
Doster, S. K., Lozano, A. M., Aguayo, A. J. & Willard, M. B. (1991) Expression of the growthassociated protein GAP-43 in adult rat retinal ganglion cells following axon injury. Neuron 6, 635–647.
Dusart, I., Airaksinen, M. S. & Sotelo, C. (1997) Purkinje cell survival and axonal regeneration are age dependent: An in vitro study. J Neurosci 17, 3710–3726.
Dusart, I., Morel, M. P., Wehrle, R. & Sotelo, C. (1999) Late axonal sprouting of injured Purkinje cells and its temporal correlation with permissive changes in the glial scar. J Comp Neurol 408, 399–418.
Dusart, I. & Schwab, M. E. (1994) Secondary cell death and the inflammatory reaction after dorsal hemisection of the rat spinal cord. Eur J Neurosci 6, 712–724.
Dusart, I. & Sotelo, C. (1994) Lack of Purkinje cell loss in adult rat cerebellum following protracted axotomy: Degenerative changes and regenerative attempts of the severed axons. J Comp Neurol 347, 211–232.
Fawcett, J. W. (1992) Intrinsic neuronal determinants of regeneration. Trends Neurosci 15, 5–8.
Fitch, M. T., Doller, C., Combs, C. K., Landreth, G. E. & Silver, J. (1999) Cellular and molecular mechanisms of glial scarringandprogressive cavitation: In vivo and in vitro analysis of inflammation-induced secondary injury after CNS trauma. J Neurosci 19, 8182–8198.
Gage, F. H., Olejniczak, P. & Armstrong, D. M. (1988) Astrocytes are important for sprouting in the septohippocampal circuit. Exp Neurol 102, 2–13.
Ghoumari, A. M., Wehrle, R., de Zeeuw, C. I., Sotelo, C. & Dusart, I. (2002) Inhibition of protein kinase C prevents Purkinje cell death but does not affect axonal regeneration. J Neurosci 22, 3531–3542.
Gianola, S. & Rossi, F. (2001) Evolution of the Purkinje cell response to injury and regenerative potential during postnatal development of the rat cerebellum. J Comp Neurol 430, 101–117.
Gianola, S. & Rossi, F. (2002) Long-term injured Purkinje cells are competent for terminal arbor growth, but remain unable to sustain stem axon regeneration. Exp Neurol 176, 25–40.
Ichikawa, R., Miyazaki, T., Kano, M., Hashikawa, T., Tatsumi, H., Sakimura, K., Mishina, M., Inoue, Y. & Watanabe, M. (2002) Distal extension of climbing fiber territory and multiple innervation caused by aberrant wiring to adjacent spiny branchlets in cerebellar Purkinje cells lacking glutamate receptor delta 2. J Neurosci 22, 8487–8503.
Inman, D., Guth, L. & Steward, O. (2002) Genetic influences on secondary degeneration and wound healing following spinal cord injury in various strains of mice. J Comp Neurol 451, 225–235.
Kaneko, T., Fujiyama, F. & Hioki, H. (2002) Immunohistochemical localization of candidates for vesicular glutamate transporters in the rat brain. J Comp Neurol 444, 39–62.
le Gal la Salle, G., Rougon, G. & Valin, A. (1992) The embryonic form of neural cell surface molecule (E-NCAM) in the rat hippocampus and its reexpression on glial cells following kainic acid-induced status epilepticus. J Neurosci 12, 872–882.
Levine, J. M. (1994) Increased expression of the NG2 chondroitin-sulfate proteoglycan after brain injury. J Neurosci 14, 4716–4730.
Liuzzi, F. J. & Lasek, R. J. (1987) Astrocytes block axonal regeneration in mammals by activating the physiological pathway. Science 237, 642–645.
Moon, L. D., Asher, R. A., Rhodes, K. E. & Fawcett, J. W. (2001) Regeneration of CNS axons back to their target following treatment of adult rat brain with chondroitinase ABC. Nat Neurosci 4, 465–466.
Nageotte, J. (1906) FrRégénération collatérale de fibres nerveuses terminées par des massues de croissance, ál'état pathologique et ál'état normal; lésions tabétiques des racinesmédullaires. Nouv. Iconographie de la Salp êtri ère 19, 217–238.
Nothias, F., Dusart, I., Roudier, F. & Peschanski, M. (1989) First month of development of fetal neurons transplanted as a cell suspension into the adult CNS. Neuroscience 33, 607–616.
Oberdick, J., Levinthal, F. & Levinthal, C. (1988) A Purkinje cell differentiation marker shows a partial DNA sequence homology to the cellular sis/PDGF2 gene. Neuron 1, 367–376.
Priller, J., Persons, D. A., Klett, F. F., Kempermann, G., Kreutzberg, G. W. & Dirnagl, U. (2001) Neogenesis of cerebellar Purkinje neurons from gene-marked bone marrow cells in vivo. J Cell Biol 155, 733–738.
Reier, P. J., Eng, L. F. & Jakeman, L. (1989) Reactive astrocyte and axonal outgrowth in the injured CNS: Is gliosis really an impediment to regeneration?. In Neural Regeneration and Transplantation (edited by Seil, F. J.) pp. 183–209, New York: Alan R Liss.
Rossi, F., Borsello, T. & Strata, P. (1994) Embryonic Purkinje cells grafted on the surface of the adult uninjured rat cerebellum migrate in the host parenchyma and induce sprouting of intact climbing fibres. Eur J Neurosci 6, 121–136.
Rossi, F., Jankovski, A. & Sotelo, C. (1995a) Differential regenerative response of Purkinje cell and inferior olivary axons confronted with embryonic grafts: Environmental cues versus intrinsic neuronal determinants. J Comp Neurol 359, 663–677.
Rossi, F., Jankovski, A. & Sotelo, C. (1995b) Target neuron controls the integrity of afferent axon phenotype: A study on the Purkinje cell-climbing fiber system in cerebellar mutant mice. J Neurosci 15, 2040–2056.
Rougon, G., Dubois, C., Buckley, N., Magnani, J. L. & Zollinger, W. (1986) Amonoclonal antibody against meningococcus group B polysaccharides distinguishes embryonic from adult N-CAM. J Cell Biol 103, 2429–2437.
Rouse, R. V. & Sotelo, C. (1990) Grafts of dissociated cerebellar cells containing Purkinje cell precursors organize into zebrin I defined compartments. Exp Brain Res 82, 401–407.
Schnell, L. & Schwab, M. E. (1993) Sprouting and regeneration of lesioned corticospinal tract fibres in the adult rat spinal cord. Eur J Neurosci 5, 1156–1171.
Schwab, M. E., Kapfhammer J. P. & Bandlow, C. E. (1993) Inhibitors of neurite growth. Ann Rev Neurosci 16, 565–595.
Sotelo, C. & Alvarado-Mallart, R. M. (1986) Growth and differentiation of cerebellar suspensions transplanted into the adult cerebellum of mice with heredodegenerative ataxia. Proc Natl Acad Sci USA 83, 1135–1139.
Sotelo, C. & Alvarado-Mallart, R. M. (1987) Embryonic and adult neurons interact to allow Purkinje cell replacement in mutant cerebellum. Nature 327, 421–423.
Spencer, R., Charman, M., Emtage, J. S. & Lawson, D. E. M. (1976) Production and properties of vitamin D-induced mRNA for chick calcium-binding protein. Eur J Biochem 71, 399–409.
Steward, O., Schauwecker, P. E., Guth, L., Zhang, Z., Fujiki, M., Inman, D., Wrathall, J., Kempermann, G., Gage, F. H., Saatman, K. E., Raghupathi, R. & Mcintosh, T. (1999) Genetic approaches to neurotrauma research: Opportunities and potential pitfalls of murine models. Exp Neurol 157, 19–42.
Tetzlaff, W., Alexander, S. W., Miller, F. D. & Bisby, M. A. (1991) Response of facial and rubrospinal neurons to axotomy: Changes in mRNA expression for cytoskeletal proteins proteins and GAP-43. J Neurosci 11, 252–2544.
Tomasiewicz, H., Ono, K., Yee, D., Thompson, C., Goridis, C., Rutishauser, U. & Magnuson, T. (1993) Genetic deletion of a neural cell adhesion molecule variant (N-CAM-180) produces distinct defects in the central nervous system. Neuron 11, 1163–1174.
Varoqui, H., Schafer, M. K., Zhu, H., Weihe, E. & Erickson, J. D. (2002) Identification of the differentiation-associatedNa+/PI transporter as a novel vesicular glutamate transporter expressed in a distinct set of glutamatergic synapses. J Neurosci 22, 142–155.
Vaudano, E., Campbell, G., Anderson, P. N., Davies, A. P., Woolhead, C., Schreyer, D. J. & Lieberman, A. R. (1995) The effects of a lesion or a peripheral nerve graft on GAP-43 upregulation in the adult rat brain: An in situ hybridization and immunocytochemical study. J Neurosci 15, 3594–3611.
Wang, L. & Denburg, J. L. (1992) A role for proteoglycans in the guidance of a subset of pioneer axons in cultured embryos of the cockroach. Neuron 8, 701–714.
Wassef, M., Chedotal, A., Cholley, B., Thomasset, M., Heizmann & Sotelo, C. (1992) Development of the olivocerebellar projection in the rat: I. Transient Biochemical compartmentation of the inferior olive. J Comp Neurol 323, 519–536.
Wassef, M., Zanetta, J. P., Brehier, A. & Sotelo, C. (1985) Transient biochemical compartmentalization of Purkinje cells during early cerebellar development. Dev Biol 111, 129–137.
Wehrle, R., Caroni, P., Sotelo, C. & Dusart, I. (2001) Role of GAP-43 in mediating the responsiveness of cerebellar and precerebellar neurons to axotomy. Eur J Neurosci 13, 857–870.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Morel, M.P., Dusart, I. & Sotelo, C. Sprouting of adult Purkinje cell axons in lesioned mouse cerebellum: “Non-permissive” versus “permissive” environment. J Neurocytol 31, 633–647 (2002). https://doi.org/10.1023/A:1025739511646
Issue Date:
DOI: https://doi.org/10.1023/A:1025739511646