Abstract
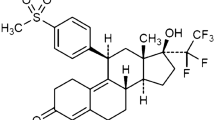
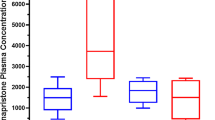
Antiprogestins represent a relatively new and promising class of therapeutic agents that could have significant impact on human health and reproduction. In the present work, the pharmacodynamics, Pharmacokinetics, and metabolism of mifepristone (MIF), lilopristone (LIL), and onapristone (ONA) in humans are reviewed, and characteristics bearing important clinical implications are discussed. Although MIF has gained notoriety as an “abortion pill,” antiprogestins may more importantly prove effective in the treatment of endometriosis, uterine leiomyoma, meningioma, cancers of the breast and prostate, and as contraceptive agents. MIF pharmacokinetics display nonlinearities associated with saturable plasma protein (α 1 -acid glycoprotein, AAG) binding and characterized by lack of dose dependency for parent drug plasma concentrations (for doses greater than 100 mg) and a zero-order phase of elimination. LIL and ONA pharmacokinetics are less well characterized but ONA does not appear to bind AAG and displays a much shorter t1/2 than the other agents. The three antiprogestins are substrates of cytochrome P450 (CYP) 3A4, an enzyme exceedingly important in human xenobiotic metabolism. Even more implicative of likely drug–drug interactions subsequent to their long-term administration are recent data from our laboratory indicating that they inactivate CYP3A4 in a cofactor- and time-dependent manner, suggesting that complexation and induction of the enzyme may occur in vivo via protein stabilization. Moreover, it has been demonstrated that MIF increases CYP3A4 mRNA levels in human hepatocytes in primary culture, indicative of message stabilization and/or transcriplional activation of CYP3A4 expression. Finally, MIF has also been shown to inhibit P-glycoprotein function. Whether LIL and ONA share these latter two characteristics with MIF has not yet been determined but they illustrate properties that, in addition to diminished antiglucocorticoid activities and altered pharmacokinetic characteristics, warrant consideration during the development of these and newer antiprogestational agents.
Similar content being viewed by others
REFERENCES
G. Pincus. The Control of Fertility, Academic Press, New York, 1965, pp. 128–138.
A. Ulmann, G. Teutsch, and D. Philibert. RU 486. Sci. Am. 262:42–48 (1990).
C. P. Puri and P. F. A. Van Look. Newly developed competitive progesterone antagonists for fertility control. In M. K. Agarwal (ed.), Antihormones in Health and Disease: Proceedings of a Satellite Symposium of the 2nd European Congress of Endocrinology, Karger, Basel, 1991, pp. 127–167.
R. Wiechert and G. Neef. Synthesis of antiprogestational steroids. J. Steroid Biochem. 27:851–858 (1987).
H. B. Croxatto, A. M. Salvatierra, B. Fuentealba, C. Zurth, and S. Beier. Effect of the antiprogestin onapristone on follicular growth in women. Hum. Reprod. 9:1442–1447 (1994).
M. L. Swahn, L. Kovacs, S. Z. Cekan, A. R. Aedo, and P. Westlun. Termination of early pregnancy with ZK 98.734: pharmacokinetic behaviour and clinical effect. Hum. Reprod. 9:57–63 (1994).
S. T. Cameron, H. O. Critchley, C. H. Buckley, T. Chard, R. W. Kelly, and D. T. Baird. The effects of post-ovulatory administration of onapristone on the development of a secretory endometrium. Hum. Reprod. 11:40–49 (1996).
R. M. Evans. The steroid and thyroid hormone receptor superfamily. Science 240:889–895 (1988).
M. Beato. Gene regulation by steroid hormones. Cell 56:335–344 (1989).
B. W. O'Malley and M. J. Tsai. Molecular pathways of steroid receptor action. Biol. Reprod. 46:163–167 (1992).
M. J. Tsai and B. W. O'Malley. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Ann. Rev. Biochem. 63:451–486 (1994).
O. Heikinheimo, K. Kontula, H. Croxatto, I. Spitz, T. Luukkainen, and P. Lähteenmäki. Plasma concentrations and receptor binding of RU 486 and its metabolites in humans. J. Steroid Biochem. 26:279–284 (1987).
D. Philibert. RU 38486: An original multifaceted antihormone in vivo. In K. Agarwal (ed.), Adrenal Steroid Antagonism, Walter de Gruyter, Berlin, 1984, pp. 77–101.
G. F. Allan, X. Leng, S. Y. Tsai, N. L. Weigel, D. P. Edwards, M. J. Tsai, and B. W. O'Malley. Hormone and antihormone induce distinct conformational changes which are central to steroid receptor activation. J. Biol. Chem. 267:19513–19520 (1992).
L. Klein-Hitpass, A. C. Cato, D. Henderson, and G. U. Ryffel. Two types of antiprogestins identified by their differential action in transcriptionally active extracts from T47D cells. Nucleic Acids Res. 19:1227–1234 (1991).
G. Neef, S. Beier, W. Elger, D. Henderson, and R. Wiechert. New steroids with antiprogestational and antiglucocorticoid activities. Steroids 44:349–372 (1984).
N. L. Weigel, C. A. Beck, P. A. Estes, P. Prendergast, M. Altmann, K. Christensen, and D. P. Edwards. Ligands induce conformational changes in the carboxyl-terminus of progesterone receptors which are detected by a site-directed antipeptide monoclonal antibody. Mol. Endocrinol. 6:1585–1597 (1992).
E. Vegeto, G. F. Allan, W. T. Schrader, M. J. Tsai, D. P. McDonnell, and B. W. O'Malley. The mechanism of RU486 antagonism is dependent on the conformation of the carboxyterminal tail of the human progesterone receptor. Cell 90:703–713 (1992).
D. el Ashry, S. A. Onate, S. K. Nordeen, and D. P. Edwards. Human progesterone receptor complexed with the antagonist RU 486 binds to hormone response elements in a structurally altered form. Mol. Endocrinol. 3:1545–1558 (1989).
M. E. Meyer, A. Pornon, J. W. Ji, M. T. Bocquel, P. Chambon, and H. Gronemeyer. Agonistic and antagonistic activities of RU486 on the functions of the human progesterone receptor. EMBO J. 9:3923–3932 (1990).
D. P. Edwards, M. Altmann, A. DeMarzo, Y. Zhang, N. L. Weigel, and C. A. Beck. Progesterone receptor and the mechanism of action of progesterone antagonists. J. Steroid Biochem. Mol. Biol. 53:449–458 (1995).
G. F. Allan, E. Lombardi, D. Haynes-Johnson, S. Palmer, M. Kiddoe, P. Kraft, C. Campen, P. Rybczynski, D. W. Combs, and A. Phillips. Induction of a novel conformation in the progesterone receptor by ZK98299 involves a defined region of the carboxyl-terminal tail. Mol. Endocrinol. 10:1206–1213 (1996).
G. S. Takimoto, D. M. Tasset, A. C. Eppert, and K. B. Horwitz. Hormone-induced progesterone receptor phosphorylation consists of sequential DNA-independent and DNA-dependent stages: analysis with zinc finger mutants and the progesterone antagonist ZK98299. Proc. Natl. Acad. Sci. U.S. 89:3050–3054 (1992).
M. T. Bocquel, J. Ji, T. Ylikomi, B. Benhamou, A. Vergezac, P. Chambon, and H. Gronemeyer. Type II antagonists impair the DNA binding of steroid hormone receptors without affecting dimerization. J. Steroid Biochem. Mol. Biol. 45:205–215 (1993).
C. A. Beck, Y. Zhang, N. L. Weigel, and D. P. Edwards. Two types of antiprogestins have distinct effects on site-specific phosphorylation of human progesterone receptor. J. Biol. Chem. 271:1209–1217 (1996).
C. A. Beck, N. L. Weigel, M. L. Moyer, S. K. Nordeen, and D. P. Edwards. The progesterone antagonist RU486 acquires agonist activity upon stimulation of cAMP signaling pathways. Proc. Natl. Acad. Sci. U.S. 90:4441–4445 (1993).
K. Delabre, M. A. Guiochon, and E. Milgrom. In vivo evidence against the existence of antiprogestins disrupting receptor binding to DNA. Proc. Natl. Acad. Sci. U.S. 90:4421–4425 (1993).
P. Kastner, A. Krust, B. Turcotte, U. Stropp, L. Tora, H. Gronemeyer, and P. Chambon. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J. 9:1603–1614 (1990).
L. L. Wei, P. Hawkins, C. Baker, B. Norris, P. L. Sheridan, and P. G. Quinn. An aminoterminal truncated progesterone receptor isoform, PRc, enhances progestin-induced transcriptional activity. Mol. Endocrinol. 10:1379–1387 (1996).
M. T. Bocquel, V. Kumar, C. Stricker, P. Chambon, and H. Gronemeyer. The contribution of the N-and C-terminal regions of steroid receptors to activation of transcription is both receptor and cell-specific. Nucleic Acids Res. 17:2581–2595 (1989).
C. A. Sartorius, L. Tung, G. S. Takimoto, and K. B. Horwitz. Antagonist-occupied human progesterone receptors bound to DNA are functionally switched to transcriptional agonist by cAMP. J. Biol. Chem. 268:9262–9266 (1993).
C. A. Sartorius, S. D. Groshong, L. A. Miller, R. L. Powell, L. Tung, G. S. Takimoto, and K. B. Horwitz. New T47D breast cancer cell lines for the independent study of progesterone B-and A-receptors: Only antiprogestin-occupied B-receptors are switched to transcriptional agonists by cAMP. Cancer Res. 54:3868–3877 (1994).
T. A. Jackson, J. K. Richer, D. L. Bain, G. S. Takimoto, L. Tung, and K. B. Horwitz. The partial agonist activity of antagonist-occupied steroid receptors is controlled by a novel hinge domain-binding coactivator L7/SPA and the corepressors N-CoR and SMRT. Mol. Endocrinol. 11:693–705 (1997).
A. Gravanis, G. Schaison, M. George, J. de Brux, P. G. Satyaswaroop, E. E. Baulieu, and P. Robel. Endometrial and pituitary responses to the steroidal antiprogestin RU 486 in postmenopausal women. J. Clin. Endocrinol. Metab. 60:156–163 (1985).
S. K. Nordeen, B. J. Bona, and M. L. Moyer. Latent agonist activity of the steroid antagonist, RU486, is unmasked in cells treated with activators of protein kinase A. Mol. Endocrinol. 7:731–742 (1993).
J. S. Mymryk and T. K. Archer. Influence of hormone antagonists on chromatin remodeling and transcription factor binding to the mouse mammary tumor virus promoter in vivo. Mol. Endocrinol. 9:1825–1834 (1995).
D. P. McDonnell and M. E. Goldman. RU486 exerts antiestrogenic activities through a novel progesterone receptor A form-mediated mechanism. J. Biol. Chem. 269:11945–11949 (1994).
N. Applezweig. Steroid Drugs, McGraw-Hill, New York, 1962, pp. 194–195.
E. E. Baulieu. RU 486—A decade on today and tomorrow. In M. Donaldson, L. Dorflinger, S. S. Brown, and L. Z. Benet (eds.), Clinical Applications of Mifepristone (RU 486) and Other Antiprogestins, National Academy Press, Washington, DC, 1993, pp. 71–119.
W. Herrmann, R. Wyss, A. Riondel, D. Philibert, G. Teutsch, E. Sakiz, and E. E. Baulieu. Effet d'un steroide anti-progesterone chez la femme: Interruption du cycle menstruel et de la grossesse au debut [The effects of an antiprogesterone steroid in women: Interruption of the menstrual cycle and of early pregnancy]. Compt. Rend. 294:933–938 (1982).
L. Kovacs, M. Sas, B. A. Resch, G. Ugocsai, M. L. Swahn, M. Bygdeman, and P. J. Rowe. Termination of very early pregnancy by RU 486—an antiprogestational compound. Contraception 29:399–410 (1984).
B. Couzinet, S. N. Le, A. Ulmann, E. E. Baulieu, and G. Schaison. Termination of early pregnancy by the progesterone antagonist RU 486 (Mifepristone). New. Engl. J. Med. 315:1565–1570 (1986).
D. J. Mishell, D. Shoupe, P. F. Brenner, M. Lacarra, J. Horenstein, P. Lähteenmäki, and I. M. Spitz. Termination of early gestation with the anti-progestin steroid RU 486: Medium vs. low dose. Contraception 35:307–321 (1987).
D. A. Grimes, D. J. Mishell, D. Shoupe, and M. Lacarra. Early abortion with a single dose of the antiprogestin RU486. Am. J. Obstet. Gynecol. 158:1307–1312 (1988).
M. Bygdeman and M. L. Swahn. Progesterone receptor blockage. Effect on uterine contractility and early pregnancy. Contraception 32:45–51 (1985).
L. Cheng, R. W. Kelly, K. J. Thong, R. Hume, and D. T. Baird. The effect of mifepristone (RU486) on the immunohistochemical distribution of prostaglandin E and its metabolite in decidual and chorionic tissue in early pregnancy. J. Clin. Endocrinol. Metab. 77:873–877 (1993).
L. Cheng, R. W. Kelly, K. J. Thong, R. Hume, and D. T. Baird. The effects of mifepristone (RU486) on prostaglandin dehydrogenase in decidual and chorionic tissue in early pregnancy. Hum. Reprod. 8:705–709 (1993).
M. W. Rodger and D. T. Baird. Induction of therapeutic abortion in early pregnancy with mifepristone in combination with prostaglandin pessary. Lancet 2:1415–1418 (1987).
M. L. Swahn and M. Bygdeman. Termination of early pregnancy with RU 486 (mifepristone) in combination with a prostaglandin analogue (sulprostone). Acta Obstet. Gynecol. Scand. 68:293–300 (1989).
E. Aubeny and E. E. Baulieu. Activite contragestive de l'association an RU486 d'une prostaglandine active par voie orale [Contraceptive activity of RU486 and oral active prostaglandin combination]. Compt. Rend. 312:539–545 (1991).
N. C. Hill, J. Ferguson, and I. Z. MacKenzie. The efficacy of oral mifepristone (RU 38.486) with a prostaglandin E1 analog vaginal pessary for the termination of early pregnancy: Complications and patient acceptability. Am. J. Obstet. Gynecol. 162:414–417 (1990).
The efficacy and tolerance of mifepristone and prostaglandin in first trimester termination of pregnancy. UK Multicentre Trial. Br. J. Obstet. Gynaecol. 97:480–486 (1990).
J. E. Norman, K. J. Thong, and D. T. Baird. Uterine contractility and induction of abortion in early pregnancy by misoprostol and mifepristone [see comments]. Lancet 338:1233–1236 (1991).
R. Peyron, E. Aubeny, V. Targosz, L. Silvestre, M. Renault, F. Elkik, P. Leclerc, A. Ulmann, and E. E. Baulieu. Early termination of pregnancy with mifepristone (RU 486) and the orally active prostaglandin misoprostol [see comments]. New Engl. J. Med. 328:1509–1513 (1993).
L. Silvestre, C. Dubois, M. Renault, Y. Rezvani, E. E. Baulieu, and A. Ulmann. Voluntary interruption of pregnancy with mifepristone (RU 486) and a prostaglandin analogue. A large-scale French experience [see comments]. New Engl. J. Med. 322:645–648 (1990).
The efficacy and tolerance of mifepristone and prostaglandin in termination of pregnancy of less than 63 days gestation; UK Multicentre Study—final results. Contraception 55:1–5 (1997).
E. A. Schaff, L. S. Stadalius, S. H. Eisinger, and P. Franks. Vaginal misoprostol administered at home after mifepristone (RU486) for abortion. J. Fam. Practice 44:353–360 (1997).
D. Jannet, N. Afiak, A. Abankwa, B. Carbonne, L. Marpeau, and J. Milliez. Termination of 2nd and 3rd trimester pregnancies with mifepristone and misoprostol. Eur. J. Obstet. Gynecol. Reprod. Biol. 70:159–163 (1996).
M. Donaldson, L. Dorflinger, S. S. Brown, and L. Z. Benet (eds.). Clinical Applications of Mifepristone (RU 486) and Other Antiprogestins, National Academy Press, Washington, DC, 1993.
M. L. Swahn, L. Kovacs, S. Z. Cekan, A. R. Aedo, and P. Westlund. Termination of early pregnancy with ZK 98.734; pharmacokinetic behavior and clinical effect. Hum. Reprod. 9:57–63 (1994).
R. Wiechert and G. Neef. Synthesis of antiprogestational steroids. J. Steroid Biochem. 27:851–858 (1987).
D. J. Gruol, M. C. Zee, J. Trotter, and S. Bourgeois. Reversal of multidrug resistance by RU 486. Cancer Res. 54:3088–3091 (1994).
D. J. Gruol and S. Bourgeois. Expression of the mdrl P-glycoprotein gene: A mechanism of escape from glucocorticoid-induced apoptosis. Biochem. Cell Biol. 72:561–571 (1994).
V. Lecureur, O. Fardel, and A. Guillouzo. The antiprogestatin drug RU 486 potentiates doxorubicin cytotoxicity in multidrug resistant cells through inhibition of P-glycoprotein function. FEBS Lett. 355:187–191 (1994).
O. Fardel, A. Courtois, B. Drenou, T. Lamy, V. Lecureur, P. Y. le Prise, and R. Fauchet. Inhibition of P-glycoprotein activity in human leukemic cells by mifepristone. Anticancer Drugs 7:671–677 (1996).
L. M. Kettel, A. A. Murphy, A. J. Morales, and S. S. Yen. Clinical efficacy of the antiprogesterone RU486 in the treatment of endometriosis and uterine fibroids. Hum. Reprod. 9(Suppl. 1):116–120 (1994).
L. M. Kettel, A. A. Murphy, A. J. Morales, A. Ulmann, E. E. Baulieu, and S. S. Yen. Treatment of endometriosis with the antiprogesterone mifepristone (RU486). Fertil. Steril. 65:23–28 (1996).
V. J. Buttram and R. C. Reiter. Uterine leiomyomata: Etiology, symptomatology, and management. Fertil. Steril. 36:433–445 (1981).
A. A. Murphy, L. M. Kettel, A. J. Morales, V. J. Roberts, and S. S. Yen. Regression of uterine leiomyomata in response to the antiprogesterone RU 486. J. Clin. Endocrinol. Metab. 76:513–517 (1993).
R. S. Carroll, D. Glowacka, and K. Dashner. Progesterone receptor expression in meningiomas. Cancer Res. 53:1312–1316 (1993).
Y. Matsuda, K. Kawamoto, K. Kiya, K. Kurisu, K. Sugiyama, and T. Uozumi. Antitumor effects of antiprogesterones on human meningioma cells in vitro and in vivo. J. Neurosurg. 80:527–534 (1994).
S. M. Grunberg. Role of antiprogestational therapy for meningiomas. Hum. Reprod. 9(Suppl. 1):202–207 (1994).
S. Bardon, F. Vignon, D. Chalbos, and H. Rochefort. RU486, a progestin and glucocorticoid antagonist, inhibits the growth of breast cancer cells via the progesterone receptor. J. Clin. Endocrinol. Metab. 60:692–697 (1985).
G. H. Bakker, H. B. Setyono, M. S. Henkelman, F. H. de Jong, S. W. Lamberts, P. van der Schoot, and J. G. Klijn. Comparison of the actions of the antiprogestin mifepristone (RU486), the progestin megestrol acetate, the LHRH analog buserelin, and ovariectomy in treatment of rat mammary tumors. Cancer Treat. Rev. 71:1021–1027 (1987).
R. L. Sutherland, R. E. Hall, G. Y. Pang, E. A. Musgrove, and C. L. Clarke. Effect of medroxyprogesterone acetate on proliferation and cell cycle kinetics of human mammary carcinoma cells. Cancer Res. 48:5084–5091 (1988).
R. T. Bowden, J. R. Hissom, and M. R. Moore. Growth stimulation of T47D human breast cancer cells by the anti-progestin RU486. Endocrinology 124:2642–2644 (1989).
G. H. Bakker, H. B. Setyono, H. Portengen, F. H. De Jong, J. A. Foekens, and J. G. Klijn. Endocrine and antitumor effects of combined treatment with an antiprogestin and antiestrogen or luteinizing hormone-releasing hormone agonist in female rats bearing mammary tumors. Endocrinology 125:1593–1598 (1989).
G. H. Bakker, H. B. Setyono, H. Portengen, F. H. De Jong, J. A. Foekens, and J. G. Klijn. Treatment of breast cancer with different antiprogestins: Preclinical and clinical studies. J. Steroid Biochem. Mol. Biol. 37:789–794 (1990).
H. Michna, M. R. Schneider, Y. Nishino, and M. F. el Etreby. Antitumor activity of the antiprogestins ZK 98.299 and RU 38.486 in hormone dependent rat and mouse mammary tumors: Mechanistic studies. Breast Cancer Res. Treat. 14:275–288 (1989).
G. Romieu, T. Maudelonde, A. Ulmann, H. Pujol, J. Grenier, G. Cavalie, S. Khalaf, and H. Rochefort. The antiprogestin RU486 in advanced breast cancer: Preliminary clinical trial. Bull. Cancer (Paris) 74:455–461 (1987).
J. G. Klijn, F. H. De Jong, G. H. Bakker, S. W. Lamberts, C. J. Rodenburg, and F. J. Alexieva. Antiprogestins, a new form of endocrine therapy for human breast cancer. Cancer Res. 49:2851–2856 (1989).
D. Perrault, E. A. Eisenhauer, K. I. Pritchard, L. Panasci, B. Norris, T. Vandenberg, and B. Fisher. Phase II study of the progesterone antagonist mifepristone in patients with untreated metastatic breast carcinoma: A National Cancer Institute of Canada Clinical Trials Group study. J. Clin. Oncol. 14:2709–2712 (1996).
O. Sartor and W. D. Figg. Mifepristone: antineoplastic studies. Clin. Obstet. Gynecol. 39:498–505 (1996).
M. F. Lin, M. H. Kawachi, M. R. Stallcup, S. M. Grunberg, and F. F. Lin. Growth inhibition of androgen-insensitive human prostate carcinoma cells by a 19-norsteroid derivative agent, mifepristone. Prostate 26:194–204 (1995).
W. L. Ledger, V. M. Sweeting, H. Hillier, and D. T. Baird. Inhibition of ovulation by low-dose mifepristone (RU 486). Hum. Reprod. 7:945–950 (1992).
H. B. Croxatto, A. M. Salvatierra, H. D. Croxatto, and B. Fuentealba. Effects of continuous treatment with low dose mifepristone throughout one menstrual cycle. Hum. Reprod. 8:201–207 (1993).
I. M. Spitz, H. B. Croxatto, A. M. Salvatierra, and O. Heikinheimo. Response to intermittent RU486 in women. Fertil. Steril. 59:971–975 (1993).
K. Gemzell-Danielsson, P. Westlund, E. Johannisson, M. L. Swahn, M. Bygdeman, and M. Seppala. Effect of low weekly doses of mifepristone on ovarian function and endometrial development. Hum. Reprod. 11:256–264 (1996).
K. Gemzell-Danielsson, M. L. Swahn, P. Svalander, and M. Bygdeman. Early luteal phase treatment with mifepristone (RU 486) for fertility regulation. Hum. Reprod. 8:870–873 (1993).
P. Dockery, R. M. Ismail, T. C. Li, M. A. Warren, and I. D. Cooke. The effect of a single dose of mifepristone (RU486) on the fine structure of the human endometrium during the early luteal phase. Hum. Reprod. 12:1778–1784 (1997).
S. T. Cameron, H. O. Critchley, C. H. Buckley, R. W. Kelly, and D. T. Baird. Effects of two antiprogestins (mifepristone and onapristone) on endometrial factors of potential importance for implantation. Fertil. Steril. 67:1046–1053 (1997).
P. Lähteenmäki, T. Rapeli, M. Kääriäinen, H. Alfthan, and O. Ylikorkala. Late postcoital treatment against pregnancy with antiprogesterone RU 486. Fertil. Steril. 50:36–38 (1988).
B. Couzinet, S. N. Le, L. Silvestre, and G. Schaison. Late luteal administration of the antiprogesterone RU486 in normal women: Effects on the menstrual cycle events and fertility control in a long-term study. Fertil. Steril. 54:1039–1044 (1990).
A. Glasier, K. J. Thong, M. Dewar, M. Mackie, and D. T. Baird. Mifepristone (RU 486) compared with high-dose estrogen and progestogen for emergency postcoital contraception [see comments]. New Engl. J. Med. 327:1041–1044 (1992).
A. M. Webb, J. Russell, and M. Elstein. Comparison of Yuzpe regimen, danazol, and mifepristone (RU486) in oral postcoital contraception. Br. Med. J. 305:927–931 (1992).
O. Heikinheimo and D. F. Archer. Mifepristone: A potential contraceptive. Clin. Obstet. Gynecol. 39:461–468 (1996).
R. Deraedt, C. Bonnet, M. Busigny, P. Chatelet, C. Cousty, M. Mouren, D. Philibert, J. Pottier, and J. Salmon. Pharmacokinetics of RU 486. In E. E. Beaulieu and S. I. Segal (eds.), The Antiprogestin Steroid RU 486 and Human Fertility Control, Plenum Press, New York, 1985, pp. 103–122.
P. Lähteenmäki, O. Heikinheimo, H. Croxatto, I. Spitz, D. Shoupe, L. Birgerson, and T. Luukkainen. Pharmacokinetics and metabolism of RU 486. J. Steroid Biochem. 27:859–863 (1987).
R. Kekkonen, O. Heikinheimo, E. Mandelin, and P. Lähteenmäki. Pharmacokinetics of mifepristone after low oral doses. Contraception 54:229–234 (1996).
E. E. Baulieu. Contragestion and other clinical applications of RU 486, an antiprogesterone at the receptor. Science 245:1351–1357 (1989).
B. Grimaldi, C. Hamberger, D. Tremblay, J. Barre, and J. P. Tillement. In vitro study of the binding of RU 486 and RU 42633 to human serum proteins. Prog. Clin. Biol. Res. 300:445–448 (1989).
O. Heikinheimo. Antiprogesterone steroid RU486. Pharmacokinetics and receptor binding in humans. Acta Obstet. Gynecol. Scand. 69:357–358 (1990).
K. Nagoshi, N. Hayashi, and K. Sekiba. Automated direct assay system for RU38486, an antiprogesterone-antiglucocorticoid agent, and its metabolites using high performance liquid chromatography. Acta Med. Okayama 45:81–87 (1991).
Y. E. Shi, Z. H. Ye, C. H. He, G. Q. Zhang, J. Q. Xu, L. P. Van, and K. Fotherby. Pharmacokinetic study of RU 486 and its metabolites after oral administration of single doses to pregnant and non-pregnant women. Contraception 48:133–149 (1993).
O. Heikinheimo. Pharmacokinetics of the antiprogesterone RU 486 in women during multiple dose administration. J. Steroid Biochem. 32:21–25 (1989).
O. Heikinheimo, S. Ranta, S. Grunberg, P. Lähteenmäki, and I. M. Spitz. Alterations in the pituitary-thyroid and pituitary-adrenal axes—consequences of long-term mifepristone treatment. Metabolism 46:292–296 (1997).
O. Heikinheimo, O. Ylikorkala, U. Turpeinen, and P. Lähteenmäki. Pharmacokinetics of the antiprogesterone RU 486: No correlation to clinical performance of RU 486. Acta Endocrinol. 123:298–304 (1990).
P. F. A. Van Look and H. Von Hertzen. Antiprogestins: Perspectives from a Global Research Program. In M. S. Donaldson, L. Dorflinger, S. S. Brown, and L. Z. Benet (eds.), Clinical Applications of Mifepristone (RU 486) and Other Antiprogestins, National Academy Press, Washington, DC, 1993, pp. 253–277.
G. R. Jang, S. A. Wrighton, and L. Z. Benet. Identification of CYP3A4 as the principal enzyme catalyzing mifepristone (RU 486) oxidation in human liver microsomes. Biochem. Pharmacol. 52:753–761 (1996).
S. A. Wrighton, M. VandenBranden, and B. J. Ring. The human drug metabolizing cytochromes P450. J. Pharmacokin. Biopharm. 24:461–473 (1996).
G. R. Jang and L. Z. Benet. Cytochrome P450 3A4 mediated N-demethylation of the antiprogestins lilopristone and onapristone. Drug Metab. Dispos. 25:1119–1122 (1997).
G. R. Jang and L. Z. Benet. Antiprogestin-mediated inactivation of cytochrome P450 3A4. Pharmacology, 56:150–157 (1998).
T. A. Kocarek, E. G. Schuetz, S. C. Stom, R. A. Fisher, and P. S. Guzelian. Comparative analysis of cytochrome P4503A induction in primary cultures of rat, rabbit, and human hepatocytes. Drug Metab. Dispos. 23:415–421 (1995).
L. Z. Benet, D. L. Kroetz, and L. B. Sheiner. Pharmacokinetics: The dynamics of drug absorption, distribution, and elimination. In J. G. Hardman, L. E. Limbird, P. B. Molinoff, R. W. Ruddon, and A. G. Goodman (eds.), Goodman and Gilman's The Pharmacological Basis of Therapeutics, McGraw-Hill, New York, 1996, chap. 1, pp. 3–27.
F. P. Guengerich, M. V. Martin, P. H. Beaune, P. Kremers, T. Wolff, and D. J. Waxman. Characterization of rat and human liver microsomal cytochrome P-450 forms involved in nifedipine oxidation, a prototype for genetic polymorphism in oxidative drug metabolism. J. Biol. Chem. 261:5051–5060 (1986).
T. Kronbach, V. Fischer, and U. A. Meyer. Cyclosporine metabolism in human liver: Identification of a cytochrome P-450III gene family as the major cyclosporine-metabolizing enzyme explains interactions of cyclosporine with other drugs. Clin. Pharmacol. Ther. 43:630–635 (1988).
M. Sattler, F. P. Guengerich, C. H. Yun, U. Christians, and K. F. Sewing. Cytochrome P-450 3A enzymes are responsible for biotransformation of FK506 and rapamycin in man and rat. Drug Metab. Dispos. 20:753–761 (1992).
T. Kronbach, D. Mathys, M. Umeno, F. J. Gonzalez, and U. A. Meyer. Oxidation of midazolam and triazolam by human liver cytochrome P450IIIA4. Mol. Pharmacol. 36:89–96 (1989).
S. Imaoka, K. Enomoto, Y. Oda, A. Asada, M. Fujimon, T. Shimada, S. Fujita, F. P. Guengerich, and Y. Funae. Lidocaine metabolism by human cytochrome P-450s purified from hepatic microsomes: comparison of those with rat hepatic cytochrome P-450s. J. Pharmacol. Exp. Ther. 255:1385–1391 (1990).
J. M. Trivier, C. Libersa, C. Belloc, and M. Lhermitte. Amiodarone N-deethylation in human liver microsomes: Involvement of cytochrome P450 3A enzymes (first report). Life Sci. 52:PL91–96 (1993).
G. Fabre, B. Julian, A. B. Saint, H. Joyeux, and Y. Berger. Evidence for CYP3A-mediated N-deethylation of amiodarone in human liver microsomal fractions. Drug Metab. Dispos. 21:978–985 (1993).
F. P. Guengerich, E. D. Muller, and I. A. Blair. Oxidation of quinidine by human liver cytochrome P-450. Mol. Pharmacol. 30:287–295 (1986).
J. W. Harris, A. Rahman, B. R. Kim, F. P. Guengerich, and J. M. Collins. Metabolism of taxol by human hepatic microsomes and liver slices: Participation of cytochrome P450 3A4 and an unknown P450 enzyme. Cancer Res. 54:4026–4035 (1994).
M. V. Relling, R. Evans, C. Dass, D. M. Desiderio, and J. Nemec. Human cytochrome P450 metabolism of teniposide and etoposide. J. Pharmacol. Exp. Ther. 261:491–496 (1992).
P. X. Zhou, E. Seree, X. J. Zhou, M. Placidi, P. Maurel, Y. Barra, and R. Rahmani. Involvement of human liver cytochrome P450 3A in vinblastine metabolism: Drug interactions. Cancer Res. 53:5121–5126 (1993).
X. J. Zhou, P. X. Zhou, T. Gauthier, M. Placidi, P. Maurel, and R. Rahmani. Human liver microsomal cytochrome P450 3A isozymes mediated vindesine biotransformation. Metabolic drug interactions. Biochem. Pharmacol. 45:853–861 (1993).
G. R. Wilkinson. Cytochrome P4503A (CYP3A) metabolism: Prediction of in vivo activity in humans. J. Pharmacokin. Biopharm. 24:475–490 (1996).
E. G. Schuetz and P. S. Guzelian. Induction of cytochrome P-450 by glucocorticoids in rat liver. II. Evidence that glucocorticoids regulate induction of cytochrome P-450 by a nonclassical receptor mechanism. J. Biol. Chem. 259:2007–2012 (1984).
L. C. Quattrochi, A. S. Mills, J. L. Barwick, C. B. Yockey, and P. S. Guzelian. A novel cis-acting element in a liver cytochrome P450 3A gene confers synergistic induction by glucocorticoids plus antiglucocorticoids. J. Biol. Chem. 270:28917–28923 (1995).
J. D. Schuetz, E. G. Schuetz, J. V. Thottassery, P. S. Guzelian, S. Strom, and D. Sun. Identification of a novel dexamethasone responsive enhancer in the human CYP3A5 gene and its activation in human and rat liver cells. Mol. Pharmacol. 49:63–72 (1996).
L. Z. Benet, C.-Y. Wu, M. F. Hebert, and V. J. Wacher. Intestinal drug metabolism and antitransport processes: A potential paradigm shift in oral drug delivery. J. Controlled Release 39:139–143 (1996).
K. S. Lown, D. G. Bailey, R. J. Fontana, S. K. Janardan, C. H. Adair, L. A. Fortlage, M. B. Brown, W. Guo, and P. B. Watkins. Grapefruit juice increases felodipine oral availability in humans by decreasing intestinal CYP3A protein expression. J. Clin. Invest. 99:2545–2553 (1997).
K. S. Lown, R. R. Mayo, A. B. Leichtman, H. L. Hsiao, D. K. Turgeon, P. Schmiedlin-Ren, M. B. Brown, W. Guo, S. J. Rossi, L. Z. Benet, and P. B. Watkins. Role of intestinal P-glycoprotein (mdr1) in interpatient variation in the oral bioavailability of cyclosporine. Clin. Pharmacol. Ther. 62:248–260 (1997).
V. J. Wacher, C. Y. Wu, and L. Z. Benet. Overlapping substrate specificities and tissue distribution of cytochrome P450 3A and P-glycoprotein: Implications for drug delivery and activity in cancer chemotherapy. Mol. Carcinogen. 13:129–134 (1995).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jang, G.R., Benet, L.Z. Antiprogestin Pharmacodynamics, Pharmacokinetics, and Metabolism: Implications for Their Long-Term Use. J Pharmacokinet Pharmacodyn 25, 647–672 (1997). https://doi.org/10.1023/A:1025725716343
Published:
Issue Date:
DOI: https://doi.org/10.1023/A:1025725716343