Abstract
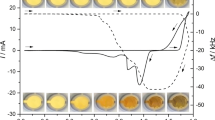
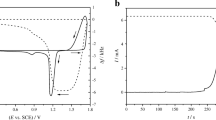
Regularities of formation of a palladium oxide layer and its cathodic reduction in 0.5 M H2SO4 at 0.5–1.3 V (SHE) are studied by cyclic voltammetry, x-ray photoelectron spectroscopy, and electrochemical quartz crystal microbalance. A pure Pd plate and a ∼0.5-μm-thick Pd coating on gold-sputtered quartz crystal is used for electrochemical and microgravimetric studies. It is shown that a Pd electrode dissolves electrochemically in 0.5 M H2SO4 when its potential is cycled between 0.5 and 1.3 V. In the case of ∼0.5-μm-thick Pd coating on the gold substrate, the decrease in the electrode weight during one anodic–cathodic cycle is 1.0–1.5 μg/cm2. It is suggested that anodic process at 0.5–1.3 V (SHE) represents electrochemical oxidation of palladium, yielding a surface layer of poorly soluble Pd(OH)2 and/or PdO phases, as expressed by the equation Pd + 2H2O ⇄ (Pd(OH)2/PdO)s + 2H+ + 2e. This surface layer, (Pd(OH)2/PdO)s, undergoes reduction during the cathodic process. About 5% of the total amount of ionized palladium dissolve in electrolyte.
Similar content being viewed by others
References
Conway, B.E., Prog. Surf. Sci., 1995, vol. 49, p. 331.
Angerstein-Kozlowska, H., Comprehensive Treaties of Electrochemistry, Yeager, E., Bockris, J.O'M., Conway, B.E., and Sarangapani, S., Eds., New York: Plenum, 1984, vol. 9, p. 22.
Bolzán, A.E., Chialvo, A.C., and Arvia, A.J., J. Electroanal. Chem., 1984, vol. 179, p. 71.
Perdriel, C.L., Custidiano, E., and Arvia, A.J., J. Electroanal. Chem., 1988, vol. 246, p. 65.
Burke, L.D. and Roche, M.B.C., J. Electroanal. Chem., 1985, vol. 186, p. 139.
Bolzán, A.E. and Arvia, A.J., J. Electroanal. Chem., 1992, vol. 322, p. 247.
Bolzán, A.E., J. Electroanal. Chem., 1995, vol. 380, p. 127.
Burke, L.D. and Casey, J.K., J. Electrochem. Soc., 1993, vol. 140, p. 1284.
Burke, L.D. and Casey, J.K., J. Electrochem. Soc., 1993, vol. 140, p. 1292.
Burke, L.D. and Casey, J.K., J. Appl. Electrochem., 1993, vol. 23, p. 573.
Burke, L.D. and Nagle, L.C., J. Electroanal. Chem., 1999, vol. 461, p. 52.
Dall'Antonia, L.H., Tremiliosi-Filho, G., and Jerkiewicz, G., J. Electroanal. Chem., 2001, vol. 502, p. 72.
Jaksic, M.M., Johansen, B., and Tunold, T., Int. J. Hydrogen Energy, 1993, vol. 18, p. 111.
Birss, V.I., Chan, M., Phan, T., Vanýsek, P., and Zhang, A., J. Chem. Soc., Faraday Trans., 1996, vol. 92, p. 4041.
Kim, K.S., Gossman, A.F., and Winograd, N., Anal. Chem., 1974, vol. 46, p. 197.
Solomun, T., J. Electroanal. Chem., 1991, vol. 302, p. 31.
Pourbaix, M., Atlas d'Equilibres Electrochimiques, Paris: Gauthier-Villars, 1963.
Jusys, Z. and Stalnionis, G., J. Electroanal. Chem., 1997, vol. 431, p. 141.
Michri, A.A., Pshenichnikov, A.G., and Burshtein R.Kh., Elektrokhim., 1972, vol. 8, p. 364.
Juodkazis, K., Juodkazyt, G., Jasiulaitiené, V., Lukinskas, A., and ebeka, B., Electrochem. Commun., 2000, vol. 2, p. 503.
Juodkazis, K., Stalnionis, G., ebeka, B., Sukiene, V., and Savickaja, I., Elektrokhim., 2002, vol. 38, p. 1283.
Juodkazis, K., Juodkazyte, J., Šebeka, B., and Lukinskas, A., Electrochem. Commun., 1999, vol. 1, p. 315.
Wagner, C.D., Riggs, W.M., Davis, L.E., Moulder, J.F., et al., Handbook of X-Ray Photoelectron Spectroscopy, Minnesota 55344: Perkin-Elmer, 1978, p. 110.
Burke, L.D. and Roche, M.B.C., J. Electroanal. Chem., 1985, vol. 186, p. 139.
Burke, L.D. and Roche, M.B.C., J. Electroanal. Chem., 1984, vol. 167, p. 291.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Juodkazis, K., Juodkazytė, J., Šebeka, B. et al. Anodic Dissolution of Palladium in Sulfuric Acid: An Electrochemical Quartz Crystal Microbalance Study. Russian Journal of Electrochemistry 39, 954–959 (2003). https://doi.org/10.1023/A:1025724021078
Issue Date:
DOI: https://doi.org/10.1023/A:1025724021078