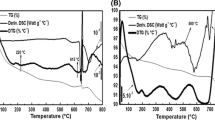
Abstract
The intercalation of methylene blue into mordenite zeolite was studied by diffuse reflectance spectroscopy. Methylene blue was incorporated into mordenite by ion exchange in the aqueous phase. Samples of sodium, calcium and protonated mordenite were subjected to methylene blue loading. The DR spectra observed shortly after mixing the dye with sodium mordenite are those of aggregated species adsorbed on the surface. The period of intercalation is very short (1 h) for protonated mordenite and is too long for calcium mordenite (7 days). The hydrated mordenite samples containing intercalated methylene blue show two 660 and 610 nm bands which are assigned to monomeric and dimeric species of methylene blue, respectively. Upon dehydration a new band at 745 nm is observed which corresponds to the protonated dye molecule. The intensity of this band increases with severity of dehydration. Those dehydrated samples containing merely aggregated dye molecules adsorbed on the surface do not show the 745 nm band. The protonation of methylene blue is reversible by the dehydration-hydration process.
Similar content being viewed by others
References
G. Schulz-Ekloff, D. Wöhrle, B.V. Duffel and R.A. Schoonheydt: Micropor. Mesopor. Mater. 51, 91 (2002).
G. Calzaferri and N. Gfeller: J. Phys. Chem. 96, 3428 (1992).
R. Hoppe, G. Schulz-Ekloff, D. Wöhrle, E.S. Shpiro and O.P. Tkachenko: Zeolites 13, 322 (1993).
W. Hölderich, G. Lauth, G. Wagenblast and E. Schefcizk: Ger. Pat. DE 4207339 assigned to W. Hölderich, 1992.
D. Wöhrle, A. Sobbi, O. Franke and G. Schulz-Ekloff: Zeolites 15, 540 (1995).
W. F. Hölderich, N. Rohrlich, P. Bartl and L. Chassot: Phys. Chem. Chem. Phys. 2, 3919 (2000).
D.E. DeVos and P.A. Jacobs: Stud. Surf. Sci. Catal. 137, 957 (2001).
D. Wöhrle and G. Schulz-Ekloff: Adv. Mater. 6, 875 (1994).
F. Bedioui: Coord. Chem. Rev. 144, 39 (1995).
M. Ehrl, H.W. Kindervater, F.W. Deeg and C. Bräuchle: J. Phys. Chem. 98, 11756 (1994).
S. Wohlrab, R. Hoppe, G. Schulz-Ekloff and D. Wöhrle: Zeolites 12, 862 (1992).
V. Ramamurthy, D.R. Sanderson and D.F. Eaton: J. Am. Chem. Soc. 115, 10438 (1993).
I. Braun, G. Schulz-Ekloff, M. Beckstette and D. Wöhrle: Zeolites 19, 128 (1997).
M. Ganschow, D. Wöhrle and G. Schulz-Ekloff: J. Porphyrins Phthalocyanine 3, 299 (1999).
M. Wark, M. Ganschow, Y. Rohlfing, G. Schulz-Ekloff and D. Wöhrle: Stud. Surf. Sci. Catal. 135, 160 (2001).
A. Ghanadzadeh and M.A. Zanjanchi: Spectrochimica Acta A 57, 1865 (2001).
B. Onida, B. Bonelli, M. Lucco-Borlera, L. Flora, C. Otero Areán and E. Garrone: Stud. Surf. Sci. Catal. 135, 364 (2001).
G.P. Handreck and T.D. Smith: J. Chem. Soc. Faraday Trans. 184, 4191 (1988).
J.R. Anderson, Y.F. Chang and A.E. Hughes: Catal. Lett. 2, 279 (1989).
M. Susic, N. Petranovic and B. Miocinovic: J. Inorg. Nucl. Chem. 34, 2349 (1972).
D.H. Park, K.W. Lee and S.J. Choe: Bull. Korean Chem. Soc. 16, 467 (1995).
Ch. Baerlocher, W.M. Meier and D.H. Olson: Atlas of Zeolite Framework Types, 5th Ed., Elsevier, 191 (2001).
E. Rabinowithch and I.F. Epstein: J. Am. Chem. Soc. 63, 69 (1941).
A.A. Shaikh, P.N. Joshi, N.E. Jacob and V.P. Shiralkar: Zeolites 13, 511 (1993).
M.M.J. Treacy and J.B. Higgins: Collection of Simulated XRDPowder Patterns for Zeolites, 4th Ed., Elsevier, 243 (2001).
R. Hoppe, G. Schulz-Ekloff, D. Wöhrle, M. Ehrl and C. Bräuchle: Stud. Surf. Sci. Catal. 69, 199 (1991).
K. Bergmann and C.T. O'konski: J. Phys. Chem. 67, 2169 (1963).
J. Cenens and K.A. Schoonheydt: Clays and Clay Minerals 36, 214 (1988).
D.W. Breck: Zeolites Molecular Sieves, Wiley-Interscience, 462 (1974).
J.W. Ward: J. Phys. Chem. 72, 4211 (1968).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zanjanchi, M., Sohrabnejad, S. Intercalation of Methylene Blue into Mordenites: Role of Zeolite Acidity. Journal of Inclusion Phenomena 46, 43–49 (2003). https://doi.org/10.1023/A:1025699425668
Issue Date:
DOI: https://doi.org/10.1023/A:1025699425668