Abstract
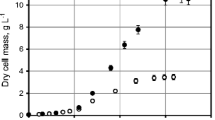
The influence of bacterial hemoglobin, VHb, on dechlorinationand degradation of 2-chlorobenzoate (2-CBA) by recombinantBurkholderia sp. under variable oxygen availability with an initial dissolved oxygenconcentration of 0.27 mM-0.72 mM was investigated in batch and continuous culture. Abilityto express VHb was provided to recombinant Burkholderia by transformationwith the VHb gene, vgb, on plasmid pSC160. 100% of 0.5 mM CBA was degraded incultures with 85% and 70% of total volume as headspace air in closed reactorsby both wild type and recombinant Burkholderia. The recombinant cultures were able todechlorinate and degrade 100% of the 2-CBA in less than 48 hours at 30 °Ccompared to more than 120 hours for wild type cultures. The rate and extent of CBAdegradation by recombinant cultures with 40% of total volume as headspace air was higher than thoseachieved by wild type cells at the end of the 168 hours of incubation period, 98and 73%, respectively. The chloride released: CBA degraded molar ratio for cultures with 40%of total volume headspace air was nearly stoichiometric (molar ratio = 1.0) for recombinantstrains, whereas it was non-stoichiometric (molar ratio = 0.24)for wild type cells. The results suggest a suicidal meta-pathway for wild type cells and a complete dechlorinationand degradation pathway for recombinant cells under hypoxic conditions.The degradation and dechlorination ability of both types of cells was alsoinvestigated in continuous reactor studies by varying the dilution rate under hypoxicconditions. Regarding potential of the recombinant strain for 2-CBA degradation in eitheropen ecosystems or closed bioreactor bioremediation systems, the stability of the plasmidcontaining vgb in the recombinant cells was also studied; the plasmid was100% stable at 0.025 h-1 dilution rate (∼1.7 d hydraulic retention time),even after one month.
Similar content being viewed by others
References
Arensdorf JJ & Focht DD (1994) Formation of chlorocatechol meta cleavage products by a Pseudomonad during metabolism of monochlorbiphenyls. Appl. Environ. Microbiol. 60: 2884–2889
Arensdorf JJ & Focht DD (1995) A meta cleavage pathway for 4-Chlorbenzoate, an intermediate in the metabolism of 4-chlorobiphenyl by Pseudomonas cepacia P166. Appl. Environ. Microbiol. 61: 443–447
Balajee S & Mahadevan A (1990) Utilization of chloroaromatic substances by Azotobacter chroococcum. Syst. Appl. Microbiol. 13: 194–198
Bartels I, Knackmuss HJ & Reineke W (1984) Suicide inactivation of catechol 2,3-dioxygenase from Pseudomonas putida mt-2 by 3-halocatechols. Appl. Environ. Microbiol. 47: 500–505
Champagne P, Van Geel, PJ & Parker WJ (1998) A proposed transient model for cometabolism in biofilm systems. Biotechnol. Bioeng. 60: 541–550
Chung JW, Webster DA, Pagilla KR & Stark BC (2001) Chromosomal integration of the Vitreoscilla hemoglobin gene in Burkholderia and Pseudomonas for the purpose of producing stable engineered strains with enhanced bioremediating ability. J. Ind. Microbiol. Biotechnol. 27: 27–33
Deweerd KA & Bedard DL (1999) Use of halogenated benzoates and other halogenated aromatic compounds to stimulate the microbial dechlorination of PCBs. Environ. Sci. Technol. 33: 2057–2063
Dikshit KL & Webster DA (1988) Cloning, characterization and expression of the bacterial globin gene from Vitreoscilla in Escherichia coli. Gene 70: 377–386
Dikshit KL, Dikshit, RP & Webster DA (1990) Study of Vitreoscilla globin (vgb) gene expression and promoter activity in E. coli through transcriptional fusion. Nucl. Acid Res. 18: 4149–4155
Fish PA, Webster DA & Stark BC (2000) Vitroeoscilla hemoglobin enhances the first step in 2,4-dinitrotoluene degradation in vitro and at low aeration in vivo. J. Mol. Cat. B 9: 75–82
Flanagan WP & May RJ (1993) Metabolite detection as evidence for naturally occurring aerobic PCB biodegradation in Hudson River sediments. Environ. Sci. Technol. 27: 2207–2212
Harkness MR, McDermott JB, Abramowicz DA, Salvo JJ, Flanagan WP, Stephens ML, Mondello FJ, May RJ, Lobos JH, Carroll KM, Brenna MJ, Bracco AA, Fish KM, Warner GL, Wilson PR, Dietrich DK, Lin DT, Morgan, CB & Gately WL (1993) In situ stimulation of aerobic PCB degradation in Hudson river sediments. Science 259: 503–507
Joshi M & Dikshit KL (1994) Oxygen dependent regulation of Vitreoscilla globin gene: evidence for positive regulation by FNR. Biochem. Biophys. Com. 202: 535–542
Khosla C & Bailey JE (1988a) The Vitreoscilla hemoglobin gene: Molecular cloning, nucleotide sequence, and genetic expression in Escherichia coli. Mol. Gen. Genetics. 214: 158–161
Khosla C & Bailey JE (1988b) Heterologous expression of a bacterial hemoglobin improves the growth properties of recombinant Escherichia coli. Nature 331: 633–635
Kröckel L & Focht DD (1987) Construction of chlorobenzene utilizing recombinants by progentive manifestation of a rare event. Appl. Environ. Microbiol. 53: 2470–2475
Krooneman J, Wieringa EBA, Moore ERB, Gerritse J, Prins RA & Gottschal, JC (1996) Isolation of Alcaligenes sp. strain L6 at low oxygen concentrations and degradation of 3-chlorobenzoate via a pathway not involving (chloro)catechols. Appl. Environ. Microbiol. 62: 2427–2434
Krooneman J, Moore ERB, van Velzen JCL, Prins RA, Forney, LJ & Gottschal, JC (1999) Isolation of Alcaligens sp. train L6 at low oxygen concentrations and degradation of 3-chlorobenzoate via a pathway not involving (Chloro) catechols. Appl. Environ. Microbiol. 62: 2427–2434
Lin JM, Stark BC & Webster DA (in press) Effects of Vitreoscilla hemoglobin on the 2,4-dinitrotoluene (DNT) dioxygenase activity of Burkholderia and on DNT degradation in two-phase bioreactors. J. Ind. Microbiol. Biotechnol.
Liu SC, Webster DA & Stark BC (1995) Cloning and expression of Vitreoscilla hemoglobin gene in Pseudomonas: Effects on cell growth. Appl. Microbiol. Biotechnol. 44: 419–424
Mars AE, Kasberg T, Kaschabek SR, van Agteren MH, Janssen DB & Reineke W (1997) Microbial degradation of chloroaromatics: use of the meta-cleavage pathway for mineralization of chlorobenzene. J. Bacteriol. 179: 4530–4537
Müller R, Deckwer WD & Hecht V (1996) Degradation of chloroand methyl-substituted benzoic acids by a genetically modified microorganism. Biotechnol. Bioeng. 51: 528–537
Nasr MA, Hwang KW, Akbas M, Webster DA & Stark BC (2001) Effects of culture conditions on enhancement of 2,4-dinitrotoluene degradation by Burkholderia engineered with the Vitreoscilla hemoglobin gene. Biotechnol. Prog. 17: 359–361
Niedan V & Schöler HF (1997) Natural formation of chlorobenzoic acids (CBA) and distinction between PCB degraded bacteria. Chemosphere 35: 1233–1241
Oldenhius R, Kuijk L, Lammers A, Janssen DB & Witholt B (1989) Degradation of chlorinated and nonchlorinated aromatic solvents in soil suspensions by pure bacterial cultures. Appl. Microbiol. Biotechnol. 30: 211–217
Oltmanns HG, Rast RH & Reineke W (1988) degradation of 1,4-dichlorobenzene and toluene by a Pseudomonas strain. Appl. Environ. Microbiol. 57: 157–162
Park KW, Kim KJ, Howard AJ, Stark BC & Webster DA (2002) Vitreoscilla hemoglobin binds to subunit I of cytochrome bo ubiquinol oxidases. J. Biol. Chem. 277: 33334–33337
Patel SM, Stark BC, Hwang KW, Dikshit KL & Webster DA (2000) Cloning and expression of Vitreoscilla hemoglobin gene in Burkholderia sp. strain DNT for enhancement of 2,4-dinitrotoluene degradation. Biotechnol. Prog. 16: 26–30
Rainer, BW (1990) Oxygen transfer in bioreactors. Chem. Biochem Eng. Q4. 4: 185–196
Ramandeep, Hwang KW, Raje M, Kim KJ, Stark BC, Dikshit KL & Webster DA (2001) Vitreoscilla hemoglobin: Intracellular localization and binding to membranes. J. Biol. Chem. 276: 24781–24789
Reineke W & Knackmuss HJ (1984) Microbial metabolism of haloaromatics: Isolation and properties of a chlorobenzene degrading bacterium. Appl. Environ. Microbiol. 47: 395–402
Romanov V & Hausinger RP (1994) Pseudomonas aeruginosa 142 uses a three-component ortho-halobenzoate 1,2-dioxygenase for metabolism of 2,4-dichloro-and 2-chlorobenzoate. J. Bacteriol. 176: 3368–3374
Schmidt E & Knackmuss H J (1980) Chemical structure and biodegradability of halogenated aromatic compounds. Conversion of chlorinated muconic acids into maleoylacetic acid. Biochem. J. 192: 339–347
Seeger M, Zielinski M, Timmis KN & Hofer B (1999) Regiospecifity of dioxygenation of di-to pentachlorobiphenyls and their degradation to chlorobenzoates by the bph-encoded catabolic pathway of Burkholderia sp. strain LB400. Appl. Environ. Microbiol. 65: 3614–3621
Spanggord RJ, Spain JC, Nishino SF & Mortelmans KE (1991) Biodegradation of 2,4-dinitrotoulene by a Pseudomonas sp. Appl. Environ. Microbiol. 57: 3200–3205
Tross ME, Schraa G & Zehinder AJB (1996) Transformation of low concentrations of 3-chlorobenzoate by Pseudomonas sp. strain B13: Kinetics and residual concentrations. Appl. Environ. Microbiol. 62: 437–442
Tsai PS, Hatzimanikatis V & Bailey JE (1996) Effect of Vitreoscilla hemoglobin dosage on microaerobic Escherchia coli carbon and energy metabolism. Biotechnol. Bioeng. 49: 139–150
Tsoi TV, Plotnikova EG, Cole J, Guerin WF, Bagdasarian M & Tiedje J (1999) Cloning, expression and nucleotide sequence of the Pseudomonas aeruginosa 142 ohb genes coding for oxygenolytic ortho dehalogenation of halobenzoates. Appl. Environ. Microbiol. 65: 2151–2162
Van Der Woude BJ (1996) Extent of reductive dechlorination of chlorobenzoates in anoxic sediment slurries depends on the sequence of chlorine removal. Environ. Science Technol. 30: 1352–1357
Van Der Woude BJ, Gottschal JC & Prins RA (1995) Degradation of 2,5-dichlorobenzoic acid by Pseudomonas aeruginosa JB2 at low oxygen tensions. Biodegradation 6: 39–46
Viliesid F & Lilly MD (1992) Influence of dissolved oxygen tension on the synthesis of catechol 1,2-dioxygenase by Pseudomonas putida. Enzyme Microbiol. Technol. 14: 561–565
Vollmer MD & Schlomann M (1995) Conversion of 2-chloro-cis,cis-muconate and its metabolites 2-chloro-and 5-chloromuconolactone by chloromuconate cycloisomerases of pJP4 and pAC27. J. Bacteriol. 177: 2938–2941
Wakabayashi S, Matsubara H & Webster DA (1986) Primary sequence of a dimeric bacterial hemoglobin from Vitreoscilla. Nature 322: 481–483
Webster D (1987) Structure and function of bacterial hemoglobin and related proteins. In: Eichorn GC & Marzilli LG (Eds) Advances in Inorganic Biochemistry, Vol 7 (pp 245–265). Elsevier, New York
Zaitsev GM, Uotila JS, Tsitko IV, Lobanok AG & Salonen MSS (1995) Utilization of halogenated benzenes, phenols and benzoates by Rhodococcus opasus GM-14. Appl. Environ. Microbiol. 61: 4191–4201
Zaitsev GM & Karasevich YN (1984) Utilization of 2-chlorobenzoic acid by Pseudomonas cepacia. Mikrobiologiya 53: 75–80
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Urgun-Demirtas, M., Pagilla, K.R., Stark, B.C. et al. Biodegradation of 2-Chlorobenzoate by Recombinant Burkholderia Cepacia Expressing Vitreoscilla Hemoglobin Under Variable Levels of Oxygen Availability. Biodegradation 14, 357–365 (2003). https://doi.org/10.1023/A:1025672528291
Issue Date:
DOI: https://doi.org/10.1023/A:1025672528291