Abstract
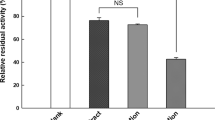
Lupeol-3-palmitate (LP) and lupeol-3-linoleate (LL), two synthetic long chain fatty acid ester analogues of the plant-derived anti-inflammatory pentacyclic triterpenoid lupeol (L), were studied in vitro as potential inhibitors of serine protease activity. With respect to the natural protein substrate bovine serum albumin (BSA), lupeol palmitate and lupeol linoleate inhibited trypsin activity in a manner consistent with mixed inhibition (KIC values of 103 and 52 μM respectively; KIU values of 30 and 14 μM respectively). However, the lupeol esters showed no inhibitory effect on the catalytic activity of porcine pancreatic elastase (PPE) with respect to the synthetic tetrapeptide substrate succinyl-(alanyl)3-p-nitroanilide (SAAANA). The present paper shows the lupeol triterpenes to be selective protease inhibitors.
Similar content being viewed by others
References
Kweifio-Okai G: Antiinflammatory activity of a Ghanaian antiarthritic herbal preparation: I. J Ethnopharmacol 33: 263-267, 1991
Kweifio-Okai G, Carroll AR: Antiarthritic effect of Alstonia boonei triterpenes. In: P. Gopalakrishnkone, C.K. Tan (eds). Recent Advances in Toxinology Research. VTRG Publishers, Singapore, 1992, pp 19-28
Kweifio-Okai G, Field B, Rumble BA, Macrides TA, De Munk F: Esterification improves the antiarthritic effectiveness of lupeol. Drug Dev Res 35: 137-141, 1995
Kweifio-Okai G, Macrides TA: Mechanisms for the antiarthritic effect of lupeol triterpenes. Can J Physiol Pharmacol 72: 272, 1994
Kweifio-Okai G, De Munk F, Macrides TA, Smith P, Rumble BA: Antiarthritic mechanisms of lupeol triterpenes. Drug Dev Res 36: 20-24, 1995
Hasmeda M, Kweifio-Okai G, Macrides TA, Poyla G: Selective inhibition of eukaryote protein kinases by anti-inflammatory triterpenoids. Planta Med 65: 14-18, 1999
Zvaifler NJ, Firestein GS: Pannus and pannocytes: Alternative models of joint destruction in rheumatoid arthritis. Arth Rheum 37: 783-789, 1994
Weiss SJ: Tissue destruction by neutrophils. N Engl J Med 320: 365-376, 1989
Mainardi CL: Biochemical mechanisms of articular destruction. Rheum Dis Clin Nth Am 13: 215-233, 1987
Kweifio-Okai G, De Munk F, Rumble BA, Macrides TA, Cropley M: Antiarthritic mechanisms of amyrin triterpenes. Res Commun Mol Pathol Pharmacol 85: 45-55, 1994
Chaudhari A, Chaturvedi AK, Parmar SS, Brumleve SJ: Antiproteolytic activity of amyrin acetate. Res Commun Chem Pathol Pharmacol 7: 205-208, 1974
Chaturvedi AK, Parmar SS, Nigam SK, Bhatnagar SC, Misra G, Sastry BVR: Anti-inflammatory and anticonvulsant properties of some natural plant triterpenoids. Pharmacol Res Commun 8: 199-210, 1976
Banerji R, Nigam SK: Anti-proteolytic activity of some triterpenoids. Int J Crude Drug Res 21: 93-95, 1983
Ying Q-L, Rinehart AR, Simon SR, Cheronis JC: Inhibition of human leukocyte elastase by ursolic acid: Evidence for a binding site for pentacyclic triterpenes. Biochem J 277: 521-526, 1991
Rajic A, Kweifio-Okai G, Macrides TA, Sandeman RM, Chandler DS, Poyla GM: Inhibition of serine proteases by anti-inflammatory triterpenes. Planta Med 66: 206-210, 2000
Facino RM, Carini M, Stefani R, Aldini G, Saibene L: Anti-elastase and anti-hyaluronidase activities of saponins and sapogenins from Hedera helix, Aesculus hippocastanum and Ruscus aculeatus: Features contributing to their efficacy in the treatment of venous insufficiency. Arch Pharm 328: 720-724, 1995
Safayhi H, Rall B, Sailer ER, Ammon HP: Inhibition by boswellic acids of human leukocyte clastase. J Pharmacol Exp Ther 281: 460-463, 1997
Ashe BM, Zimmerman M: Specific inhibition of human granulocyte elastase by cis-unsaturated fatty acids and activation by the corresponding alcohols. Biochem Biophys Res Commun 75: 194-199, 1977
Cook L, Ternai B: Similar binding sites for unsaturated fatty acids and alkyl 2-pyrone inhibitors of human sputum elastase. Biol Chem Hoppe Seyler 369: 627-631, 1988
Markwell MA, Haas SM, Bieber LL, Tolbert NE: A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem 87: 206-210, 1978
Beith J, Spiess B, Wermouth CG: The synthesis and analytical use of a highly sensitive and convenient substrate of elastase. Biochem Med 11: 350-357, 1974
Geiger R: Elastases. In: H.U. Bergmeyer (ed). Methods of Enzymatic Analysis. Verlag Chemie GmbH, Germany, 1983, pp 170-184
Steiner RF, Roth J, Robbins J: The binding of thyroxine by serum albumin as measured by fluorescence quenching. J Biol Chem 241: 560-567, 1966
Segel IH: Biochemical Calculations. John Wiley & Sons, New York, 1968, pp 366-396
Eisenthal R, Cornish-Bowden A: The direct linear plot: A new graphical procedure for estimating enzyme kinetic parameters. Biochem J 139: 715-720, 1974
Cornish-Bowden A, Wharton CW: Inhibition of enzyme activity. In: D. Rickwood (ed). Enzyme Kinetics. IRL Press, Oxford, 1988, pp 35-41
Morrisett JD, Pownall HJ, Gotto AM: Bovine serum albumin: Study of the fatty acid and steroid binding sites using spin-labelled lipids. J Biol Chem 250: 2478-2494, 1975
Nielsen AD, Borch K, Westh P: Thermochemistry of the specific binding of C12 surfactants to bovine serum albumin. Biochim Biophys Acta 1479: 321-331, 2000
Guyton AC, Hall JE: Human Physiology and Mechanisms of Disease, 6th edn. WB Saunders Company, Philadelphia, 1997, p 135
Mitaine-Offer AC, Hornebeck W, Sauvain M, Zeches-Hanrot M: Triterpenes and phytosterols as human leucocyte elastase inhibitors. Planta Med 68: 930-932, 2002
Groutas WC, Abrams WR, Theodorakis MC, Kasper AM, Rude SA, Badger RC, Ocain TD, Miller KE, Moi MK, Brubaker MJ, Davis, KS, Zandler ME: Amino acid derived latent isocyanates: Irreversible inactivation of pancreatic elastase and human leukocyte elastase. J Med Chem 28: 204-209, 1985
Bode W, Meyer E Jr, Powers JC: Human leukocyte and porcine pancreatic elastase: X-ray crystal structures, mechanism, substrate specificity, and mechanism-based inhibitors. Biochemistry 28: 1951-1963, 1989
Geetha T, Varalakshmi P: Effect of lupeol and lupeol linoleate on lysosomal enzymes and collagen in adjuvant-induced arthritis in rats. Mol Cell Biochem. 201: 83-87, 1999
Latha RM, Lenin M, Rasool M, Varalakshmi P: A novel derivative pentacyclic triterpene and omega 3 fatty acid. Prostaglandins Leukot Essent Fatty Acids. 64: 81-85, 2001
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hodges, L.D., Kweifio-Okai, G. & Macrides, T.A. Antiprotease effect of anti-inflammatory lupeol esters. Mol Cell Biochem 252, 97–101 (2003). https://doi.org/10.1023/A:1025569805468
Issue Date:
DOI: https://doi.org/10.1023/A:1025569805468