Abstract
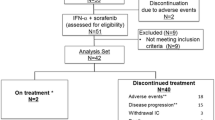
Eighteen patients with advanced renal cell carcinoma (RCC) were treated on a phase II trial with ZD1839 (IRESSA). Treatment efficacy was determined using the endpoint of improvement in time to progression (TTP) compared to TTP in patients who received interferon-α therapy. The Memorial Sloan Kettering Cancer Center database of renal cell patients shows that patients receiving therapy with interferon-α had a median TTP of 4.7 months, with 40% of patients having disease progression at 4 months. To show efficacy in this trial, 60% of evaluable patients would have to remain progression free at 4 months. Treatment with ZD1839 did not result in any complete or partial responses, and 13 patients (81%) had progression of disease within 4 months of start of therapy. At the dose and schedule used in this trial, the lack of antitumor activity associated with ZD1839 does not support further study in patients with metastatic RCC.
Similar content being viewed by others
References
Motzer RJ, Bander N, Nanus, D: Renal-cell carcinoma. N Engl J Med 335: 865–867, 1996
Korn EL, Arbuck SG, Pluda JM et al.: Clinical trial designs for cytostatic agents: are new approaches needed? J Clin Onc 19(1): 265–272, 2001
Lager DJ, Slagel DD, Palechek PL: The expression of epidermal growth factor receptor and transforming growth factor α in renal cell carcinoma. Mod Path 7: 544–548, 1994
Wells, A: Molecules in focus EGFR receptor. Int J Biochem Cell Biol 31: 637–642, 1999
Perry, JE, Grossman, ME, Tindal, DJ: Epidermal growth factor induces cyclin D1 in human prostate cancer cell line. Prostate 35: 117–124, 1998
Noonberg, SB, Benz, CC: Tyrosine kinase inhibitors targeted to the epidermal growth factor receptor subfamily. Drugs 59: 753–767, 2000
Sargent EF, Gomella LG, Bellegegrun et al.: Epidermal growth factor receptor gene expression in normal kidney and renal cell carcinoma. J Urol 142: 1364–1368, 1989
Ishikawa J, Maeda S, Umezu K: Amplification and overexpression of the epidermal growth factor receptor gene in human renal cell carcinoma. Int J Cancer 45: 1018–1021, 1990
Freeman MR, Waschecka R, Chung LWK: Aberrant expression of epidermal growth factor receptor and HER-2 (erB-2) messenger RNAs in human renal cancers. Cancer Res 49: 6221–6225, 1989
Moch H, Sauter G, Buchholz N et al.: Epidermal growth factor receptor expression is associated with rapid tumor cell proliferation in renal cell carcinoma. Hum Pathol 29: 1255–1259, 1997
Wakeling AE, Barker AJ, Davies DH, Brown DS, Green LR, Cartlidge SA, and Woodburn JR: Specific inhibition of epidermal growth factor receptor tyrosine kinase by 4–anilinoquinazolines. Breast Cancer Res Treat 38: 67–73, 1996
Ranson M, Hammond LA, Ferry D, Kris M, Tullo A, Murray PI, Miller V, Averbuch S, Ochs J, Morris C, Feyereislova A, Swaisland H, Rowinsky EK: ZD1839, a selective oral epidermal growth factor receptor-tyrosine kinase inhibitor, is well tolerated and active in patients with solid, malignant tumors: results in a phase I trial. JCO 20: 2240–2250, 2002
Janne PA, Ostler PA, Lucca J, Fidias P, Skarin AT, Lynch TJ, Johnson BE: ZD1839 (“Iressa”) show antitumor activity in patients with recurrent non-small cell lung cancer treated on a compassionate use protocol. Abstract #1274, Annual Meeting of the American Society of Clinical Oncology, 2002
Ruckdeschel JC, Simon G, Antonia S, Haura E, Williams C, Wagner H, Rocha Lima C, Ettienne K, Vaughn J, Bepler, G, Lee H: ZD1839 (“Iressa”) as a single agent for the treatment of metastatic non-small-cell lung cancer. Abstract #1269, Annual Meeting of the American Society of Clinical Oncology, 2002
Soto Parra HJ, Cavina R, Campagnoli E, Zucali P, Ginnani V, Lateri F, Ferrari B, Pedicine V, Roncalli M, Santoro A: ZD1839 (Iressa) in progressive pretreated non-small-cell lung cancer (NSCLC): reults from compassionate use in patients at the Istituto Clinico Humanitas, Rozzano, Milano. Abstract #2657, Annual Meeting of the American Society of Clinical Oncology, 2002
Motzer RJ, Mazumdar M, Bacik J et al.: Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol 17: 2530–2540, 1999
Motzer RJ, Bacik J, Murphy B, Russo P, Mazumdar, M: Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol 20: 289–296
Yang JC, Haworth L, Steinberg SM, Rosenberg SA, Novotny W: A randomized double-blind placebo-controlled trial of bevacizumab (anti-VEGF antibody) demonstrating a prolongation in time to progression in patients with metastatic renal cancer. Abstract #15, Annual Meeting of the American Society of Clinical Oncology, 2002
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Drucker, B., Bacik, J., Ginsberg, M. et al. Phase II trial of ZD1839 (IRESSA™) in patients with advanced renal cell carcinoma. Invest New Drugs 21, 341–345 (2003). https://doi.org/10.1023/A:1025472712456
Issue Date:
DOI: https://doi.org/10.1023/A:1025472712456