Abstract
Purpose. To examine the effect of long-term administration of L-carnitine on L-carnitine transport in renal brush-border membrane vesicles (BBMVs) from normotensive, Wistar-Kyoto rats.
Methods. Rats (n = 20) were orally administered 0.2 g carnitine/kg body weight per day for a total period of 8 weeks. Kinetic parameters of L-carnitine uptake were calculated by non-linear regression, and the relative abundance of the carnitine transporter, OCTN2, was determined by Western blot analysis.
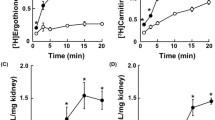
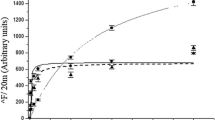
Results. Initial rates and maximal overshoot levels of Na+-dependent L-carnitine transport were significantly reduced in BBMVs from L-carnitine-treated rats compared with untreated animals. Similarly, the maximal transport rate (Vmax) of OCTN2 was lower in treated rats. However, no differences were observed in the Michaelis constant (K m) or the diffusion constant (K d) between the two groups of animals. The amount of OCTN2 protein was also decreased in L-carnitine-fed rats, this reduction being similar to that of the V max. These results were accompanied by an increase in the serum levels and also in the renal excretion of both free and esterified carnitine in treated rats, indicating that the long-term administration of L-carnitine leads to increased renal carnitine clearance.
Conclusion. These findings suggest a downregulation of OCTN2 at the renal level, in the presence of high levels of carnitine.
Similar content being viewed by others
REFERENCES
V. Tanphaichitr, D. W. Horne, and H. P. Broquist. Lysine, a precursor of carnitine in the rat. J. Biol. Chem. 246:6364-6366 (1971).
A. L. Carter, T. O. Abney, and D. F. Lapp. Biosynthesis and metabolism of carnitine. J. Child Neurol. 10:23-27 (1995).
E. P. Brass. Carnitine transport, In R. Ferrari, S. Dimauro, and G. Shewoord (eds.), L-Carnitine and its Role in Medicine: From Function to Therapy 73:287-297 (2001).
I. Tamau, R. Ohashi, J. I. Nezu, Y. Sau, D. Kobayashi, A. Oku, M. Shimane, and A. Tsuji. Molecular and functional characterization of organic cation/carnitine transporter family in mice. J. Biol. Chem. 275:40064-40072 (2000).
B. Stieger, B. O'Neill, and S. Krähenbühl. Characterization of L-carnitine transport by rat kidney brush-border membrane vesicles. Biochem. J. 309:643-647 (1995).
W. Huang, S. N. Shaikh, M. E. Ganapathy, U. Hopfer, F. H. Leibach, A. Lee Carter, and V. Ganapathy. Carnitine transport and its inhibition by sulfonylureas in human kidney proximal tubular epithelial cells. Biochem. Pharmacol. 58:1361-1370 (1999).
K. Lahjouji, C. Malo, G. A. Mitchell, and I. A. Qureshi. L-carnitine transport in mouse renal and intestinal brush-border and basolateral membrane vesicles. Biochim. Biophys. Acta 1558:82-93 (2002).
I. Tamau, K. China, Y. Sau, D. Kobayashi, J. I. Nezu, E. Kawahara, and A. Tsuji. Na+-coupled transport of L-carnitine via high-affinity carnitine transporter OCTN2 and its subcellular localization in kidney. Biochim. Biophys. Acta 1512:273-284 (2001).
W. R. Treem, C. A. Stanley, D. N. Finegold, D. E. Hale, and P. M. Coaters. Primary carnitine deficiency due to a failure of carnitine transport in kidney, muscle, and fibroblasts. N. Engl. J. Med. 319:1331-1336 (1988).
M. Horiuchi, K. Kobayashi, S. Yamaguchi, N. Shimizu, T. Koizumi, H. Nikaido, J. Hayakawa, M. Kuwajima, and T. Saheki. Primary defect of juvenile visceral steatosis (jvs) mouse with systemic carnitine deficiency is probably in renal carnitine transport system. Biochim. Biophys. Acta 1226:25-30 (1994).
V. Tanphaichitr and P. Leelahagul. Carnitine metabolism and human carnitine deficiency. Nutrition 9:246-254 (1993).
T. C. Vary and J. R. Neely. A mechanism for reduced myocardial carnitine levels in diabetic animals. Am. J. Physiol. 242:H154-H158 (1982).
A. Gürlek, E. Tutar, E. Akcil, I. Dincer, C. Erol, P. A. Kocatürk, and D. Oral. The effects of L-carnitine treatment on left ventricular function and erythrocyte superoxide dismutase activity in patients with ischemic cardiomyopathy. Eur. J. Heart Fail. 2:189-193 (2000).
M. Fujiwana, T. Nakano, S. Tamoto, and Y. Yamada. Effect of L-carnitine in patients with ischemic heart disease. J. Cardiol. 21:493-504 (1991).
H. Rauchová, Z. Dobesová, Z. Drahota, J. Zicha, and J. Kunes. The effect of chronic l-carnitine treatment on blood pressure and plasma lipids in spontaneously hypertensive rats. Eur. J. Pharmacol. 342:235-239 (1998).
O. H. Wieland, T. Deufel, and I. Paetzke-Brunner. Free and esterified carnitine: Colorimetric method. In H. U. Bergmeyer (ed.), Methods in Enzymatic Analysis, Academic Press, New York, 1985, pp. 481-488.
R. S. Hare. Endogenous creatinine in serum and urine. Pro. Soc. Exp. Biol. Med. 74:148-152 (1950).
A. Mate, M. A. de la Hermosa, A. Barfull, J. M. Planas, and C. M. Vázquez. Characterization of D-fructose transport by rat kidney brush-border membrane vesicles: changes in hypertensive rats. Cell. Mol. Life Sci. 58:1-7 (2001).
M. M. Bradford. A rapid and sensitive method for the quantitation of microgram quantities of protein utilising the principle of protein-dye binding. Anal. Biochem. 72:248-254 (1976).
J. P. Bretaudiere, A. Vassault, L. Amsellem, M. L. Pourc, H. Thieu-Phung, and M. Bailly. Criteria for establishing a standardized method for determining alkaline phosphatase activity in human serum. Clin. Chem. 23:2263-2274 (1977).
B. Colas and S. Maroux. Simultaneous isolation of brush border and basolateral membrane from rabbit enterocytes. Biochim. Biophys. Acta 600:406-420 (1980).
R. J. Pennington. Biochemistry of dystrophic muscle. Mitochondrial succinate-tetrazolium reductase and adenosine triphosphatase. Biochem. J. 80:649-652 (1961).
D. R. Absolom. Basic methods for the study of phagocytosis. Methods Enzymol. 132:95-180 (1986).
A. G. Engel, C. J. Rebouche, D. M. Wilson, A. M. Glasgow, C. A. Romshe, and R. P. Cruse. Primary systemic carnitine deficiency: II. Renal handling of carnitine. Neurology 31:819-825 (1981).
C. J. Rebouche and D. L. Mack. Sodium gradient-stimulated transport of l-carnitine into renal brush border membrane vesicles: kinetics, specificity, and #x00AEulation by dietary carnitine. Arch. Biochem. Biophys. 235:393-402 (1984).
M. Spaniol, H. Brooks, L. Auer, A. Zimmermann, M. Solioz, B. Stieger, and S. Krähenbühl. Development and characterization of an animal model of carnitine deficiency. Eur. J. Biochem. 268:1876-1887 (2001).
P. G. Welling, J. H. Thomsen, A. L. Shug, and F. L. Tse. Pharmacokinetics of L-carnitine in man following intravenous infusion of dl-carnitine. Int. J. Clin. Pharmacol. Biopharm. 17:56-60 (1979).
T. Kalaiselvi and C. Panneerselvam. Effect of L-carnitine on the status of lipid peroxidation and antioxidants in aging rats. J. Nutr. Biochem. 9:575-581 (1998).
S. Berardi, B. Stieger, B. Hagenbuch, E. Carafoli, and S. Krähenbühl. Characterization of L-carnitine transport into rat skeletal muscle plasma membrane vesicles. Eur. J. Biochem. 267:1985-1994 (2000).
I. Tamau, R. Ohashi, J. I. Nezu, H. Yabuuchi, A. Oku, M. Shimane, Y. Sau, and A. Tsuji. Molecular and functional identification of sodium ion-dependent, high affinity human carnitine transporter OCTN2. J. Biol. Chem. 273:20376-20383 (1998).
F. Scaglia, Y. Wang, and N. Longo. Functional characterization of the carnitine transporter defective in primary carnitine deficiency. Arch. Biochem. Biophys. 364:99-106 (1999).
R. Ohashi, I. Tamau, H. Yabuuchi, J. I. Nezu, A. Oku, Y. Sau, M. Shimane, and A. Tsuji. Na+-dependent carnitine transport by organic cation transporter (OCTN2): Its pharmacological and toxicological relevance. J. Pharmacol. Exp. Ther. 291:778-784 (1999).
Y. Wang, T. A. Meadows, and N. Longo. Abnormal sodium stimulation of carnitine transport in primary carnitine deficiency. J. Biol. Chem. 275:20782-20786 (2000).
C. A. Wagner, U. Lükeville, S. Kaltenbach, I. Moschen, A. Bröer, T. Risler, S. Bröer, and F. Lang. Functional and pharmacological characterization of human Na+-carnitine cotransporter hOCTN2. Am. J. Physiol. 279:F584-F591 (2000).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gómez-Amores, L., Mate, A. & Vázquez, C.M. L-Carnitine Transport in Kidney of Normotensive, Wistar–Kyoto Rats: Effect of Chronic L-Carnitine Administration. Pharm Res 20, 1133–1140 (2003). https://doi.org/10.1023/A:1025080426970
Issue Date:
DOI: https://doi.org/10.1023/A:1025080426970