Abstract
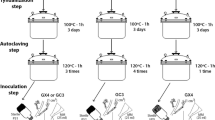
Media with different air filled porosity were compared with standard agar medium for root induction and root elongation for two Australian plants Grevillea thelemanniana and Verticordia plumosa×Chamelaucium uncinatum. Microcuttings from shoot cultures were pulsed for 7 days on a high auxin (40 μM IBA), agar-solidified medium in the dark. The rooting of the microcuttings was then compared on standard agar medium (M1, 1/2 MS, no hormones) and on three experimental treatments: – porous-agar medium (1/2 MS, no hormones, 30 g agar l−1, solidified then blended to provide aeration); – white sand, or white sand wet with M1 medium; and – a sterile propagation mix. The protocol using the propagation mix is referred to as IVS (In Vitro Soil). A separate experiment involved flushing the IVS soil profile with low or normal oxygen. The controls on M1 medium showed low and variable rooting percentages. The rate of root induction and the average total root length per microcutting at final harvest was significantly higher using the IVS protocol, porous-agar or white sand, while addition of agar medium to sand suppressed the percentage rooting and elongation as did flushing the air space in the IVS rooting medium with low oxygen. Other species tested on M1 medium and IVS including Pimelea physodes, Conospermum eatoniae, Verticordia grandis, and a Chamelaucium megalopetalum×C. uncinatum hybrid all showed a significant improvement on the IVS system. The IVS culture technique reduces plant-handling costs.
Similar content being viewed by others
References
Anderson WC, Meagher GW & Nelson AG (1977) Cost of prop-agating broccoli plants through tissue culture. HortScience 12: 543–544
Barnes LR (1979) In vitro propagation of Watermelon. Sci. Hort. 11: 223–227
Barrett-Lennard EG & Dracup M (1988) A porous-agar medium for improving the growth of plants under sterile conditions. Plant Soil 108: 204–298
Ben-Jaacov J & Dax E (1981) In vitro propagation of Grevillea rosmarinifolia. HortScience 16: 309–310
Blazich FA (1988) Mineral nutrition and adventitious rooting. In: Davis TD, Haissig BE & Sankhla N (eds) Adventitious Root Formation in Cuttings Vol. 2 (pp. 61–69). Dioscorides Press, Portland, Oregon
Bunn E & Dixon KW (1992) In vitro propagation of the rare and endangered Grevillea scapigera (Proteaceae). HortScience 27: 261–262
Debergh PC (2000) Preparation of microplants for ex vitro establishment. Acta Hortic. 530: 269–275
Debergh PC & Maene L (1981) A scheme for commercial propagation of ornamental plants by tissue culture. Sci. Hortic. 14: 335–345
De Klerk G (2002) Rooting of microcuttings: theory and practice. In Vitro Cell Dev. Biol. Plant. 38: 415–422
Douglas GC, Rutledge CB, Casey AD & Richardson DHS (1989) Micropropagation of floribunda, ground cover and minature roses. Plant Cell Tiss. Org. Cult. 19: 55–64
Gangopadhyay G, Das S, Mitra SK, Poddar R, Modak BK & Mukherjee KK (2002) Enhanced rate of multiplication and rooting through the use of coir in aseptic liquid culture media. Plant Cell Tiss. Org. Cult. 68: 301–310
Gebhardt K (1985) Development of a sterile cultivation system for rooting of shoot tip cultures (Red Rasberries) in duroplast foam. Plant Sci. 39: 141–148
Gebhardt K & Friedrich M (1987) Micropropagation of Calluna vulgaris cv. 'H.E. Beale'. Plant Cell Tiss. Org. Cult. 9: 137–145
George EF & Sherrington PD (1993) In: Plant Propagation by Tissue Culture.Part 1, The Technology., Exegentics Ltd, UK
Gorst JR, Bourne RA, Hardaker SE, Richards AE, Dircks S & de Fossard R (1978) Tissue culture propagation of two Grevillea hybrids. Int. Plant Prop. Soc. 28: 435–446
Hartmann HT, Kester DE, Davies FT & Geneve RL (1997) Plant Propagation, Principles and Practice. Prentice Hall
Handreck K & Black N (1991) Growing Media for Ornamental Plants and Turf (pp. 361–363). New South Wales University Press
Hutchinson JF, Beardsell DV & McComb J (1985) Propagation by tissue culture I. Introduction. In: Lamont B & Watkins P (eds) Horticulture of Australian Plants (pp. 38–51). The Western Australian Department of Agriculture, Perth
James DJ & Thurbon IJ (1979) Rapid in vitro rooting of apple rootstock M.9. J. Hort. Sci. 54: 309–311
Jay-Allemand C, Capelli P & Cornu D (1992) Root development of in vitro hybrid walnut microcuttings in a vermiculite-containing gelrite medium. Sci. Hortic. 51: 335–342
Karhu ST (1997) Rooting of blue honeysuckle microshoots. Plant Cell Tiss. Org. Cult. 48: 153–159
Kim M, Klopfenstein NB & Cregg BM (1989) In vitro and ex vitro rooting of micropropagated shoots using three green ash (Frax-inus pennsylvanica) clones. New For. 16: 43–47
Kirdmanee C, Kitaya Y & Kozai T (1995) Effects of oxygen enrichment and supporting material in vitro on photoautotrophic growth of Eucalyptus plantlets in vitro and ex vitro: anatomical comparisons. Acta Hortic. 393: 111–118
Kretzschmar U (1993) Improvement of Larch micropropagation by induced short shoot elongation in vitro. Silvae Genet. 42: 163–169
Lin X, Bergann BA & Stomp A (1995) Effect of medium physical support, shoot length and genotype on in vitro rooting and plantlet morphology of Sweetgum. J. Environ. Hort. 13: 117–121
McComb JA & Newton S (1980) Propagation of Kangaroo Paw using tissue culture. J. Hort. Sci. 56: 181–183
McCown BH (1988) Adventitious rooting of tissue cultured plants. In: Davis TO, Haissig BE & Sankhala N (eds) Adventitious Root Formation in Cuttings (pp. 289–302). Dioscorides Press, USA
Mohammed GH & Vidaver WE (1988) Root production and plantlet development in tissue cultured conifers. Plant Cell Tiss. Org. Cult. 14: 137–160
Mullins KV (1987) Micropropagation of Chestnut (Castanea sativa Mill.). Acta Hortic. 212: 525–520
Murashige T & Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15: 473–497
Offord CA & Campbell LC (1992) Micropropagation of Telopea speciosissima R. Br. (Proteaceae). 2: Rhizogenesis and ac-climatisation to ex vitro conditions. Plant Cell Tiss. Org. Cult. 29: 223–230
Pierik RLM (1987) In vitro Culture of Higher Plants (206). Martinus Nijhoff Publishers
Pruski KW, Lewis T, Astatkie T & Nowak J (2000) Micropropaga-tion of Chokecherry and Pincherry cultivars. Plant Cell Tiss. Org. Cult. 63: 93
Rancillac M, Faye M & David A (1982) In vitro rooting of cloned shoots in Pinus pinaster. Physiol. Plant. 56: 97–101
Roberts AV, Smith EF & Mottley J (1990) The preparation of micropropagated plantlets for transfer to soil without acclimati-zation. In: Pollard JW & Walker JM (eds) Methods in Molecular Biology. Plant Cell and Tissue CultureVol. 6 (pp. 314–322). The Humana Press
Roberts AV, Smith EF, Horan I, Walker S, Matthews D & Mottely J (1994) Stage III techniques for improving water relations and autotrophy in micropropagated plants. In: Lumsden PJ, Nicholas JR & Davies WJ (eds) Physiology, Growth and Development of Plants in Culture (pp. 314–322). Kluwer Academic Publishers
Roche TD, Long RD, Sayegh AJ & Hennerty MJ (1996) Commercial scale photo-autotrophic micropropagation applications in Irish agriculture, horticulture and forestry. Acta Hort. 440: 515–520
Rugini E & Verma DC (1982) Micropropagation of difficult to propagate almond (Prunus amygalus Batsch) cultivar. Pl. Sci. Lett. 28: 273–281
Sanchez C, San-Jose MC, Ferro E, Ballester A & Vietez AM (1997) Improved micropropagation conditions for adult-phase shoots of chestnut. J. Hort. Sci. 72: 433–443
Seaton KA & Webb MG (1996) Development of Conospermum for cut flower markets. In: Rebola K (ed) Fourth National Workshop for Australian Native Flowers (pp. 191–196). UWA Extension Conference and Seminar Management. The University of West-ern Australia, Perth
Sedgley M (1996) Banksia, Family Proteaceae. In: Johnson KJ & Burchett M (eds) Native Australian Plants - Horticulture and Uses (pp. 18–35). University of New South Wales Press, Australia
Soffer H & Burger DW (1988) Effects of dissolved oxygen con-centrations in aero hydroponics on the formation and growth of adventitious roots. J. Am. Soc. Hortic. Sci. 113: 218–221
Taji AM & Williams M (1990) Propagation of Australian plants by tissue culture. Aust. Hort., Sept (pp. 89–92).
Taji AM & Williams M (1996) Tissue Culture of Australian Plants. University of New England Printery
Van der Meeren P, De Vleeschauwer D & Debergh P (2001) Determination of oxygen profiles in agar-based gelled in vitro plant tissue culture media. Plant Cell Tiss. Org. Cult. 65: 239–245
Watad AA, Ben-Jaacov J, Tal E & Solomon H (1992) In vitro propagation of Grevillea species. Acta Hortic. 316: 51–53
Williams RR, Taji AM & Bolton JA (1985) Specificity and interaction among auxins, light, and pH in rooting of Australian woody species in vitro. HortScience 20: 1052–1053
Williams RR, Mungall ND & Taji AM (1992) Acclimatization: changes in photosynthetic competence. Acta Hortic. 314: 131–137
Zimmerman RH (1988) Micropropagation of woody plants: post tissue culture aspects. Acta Hortic. 227: 489–499
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Newell, C., Growns, D. & McComb, J. The influence of medium aeration on in vitro rooting of Australian plant microcuttings. Plant Cell, Tissue and Organ Culture 75, 131–142 (2003). https://doi.org/10.1023/A:1025072922054
Issue Date:
DOI: https://doi.org/10.1023/A:1025072922054