Abstract
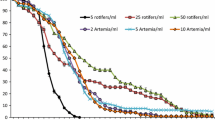
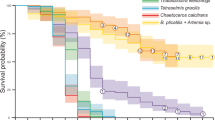
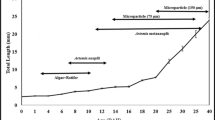
Natural populations of alligator gar (Atractosteus spatula) have declined recentlydue to the effects of commercial and sportfisheries. Aquaculture represents a short-termalternative to restore natural populations, anda first step to accomplish culture of thisspecies is the study of early life stages.Therefore, multidisciplinary research was usedto describe the major morpho-physiologicalchanges taking place during this period. Thestudies serve as a basis for the introductionof artificial diets for culture. Amorphological study distinguished differentnutritional stages, as well as externalindicators of starvation. A histologicalapproach showed the digestive tract to becompletely formed 5 days after hatching (DAH),at the beginning of exogenous feeding.Throughout larval development, intestinalmaturation was followed and a nutritionalindicator based on the mid-gut cell height wasvalidated. The occurrence of pepsin-likeproteolytic activity was detected from fiveDAH, while trypsin, chimiotrypsin andaminopeptidase-like activities graduallyincreased from two to nine DAH. The incidenceof cannibalism in culture conditions wascontrolled by exposure to anti-thyroidcompounds (thiourea – TU) to retard snoutgrowth. This treatment did not effect growthand allowed juveniles to feed on live prey butprevented the consumption of gar larvae of thesame size. Larvae exposed to3,3′,5-triiodo-1-thyronine (T3) had fasterdevelopment, a potentially advantageouscharacteristic for the repopulation of theirnatural habitat. Finally, artificial feeds werewell accepted and resulted in growth ratessimilar to those of gar larvae that were fednatural prey. This has allowed the developmentof a feeding strategy that effectively reducedcannibalism and led to the production of 30 cmjuveniles in four months.
Similar content being viewed by others
References
Anson, M.L. (1938) The estimation of pepsin, trypsin, papain and cathepsin with hemoglobin. J. Gen. Physiol. 22, 79-89.
Buddington, R.K. (1985) Digestive secretions of lake sturgeon Acipenser fulvescens during early development. J. Fish. Biol. 26, 715-724.
Buddington, R.K. and Dorshov, S.I. (1986) Development of digestive secretions in white sturgeon juveniles (Acipenser transmontanus). Comp. Biochem. Physiol. 83A, 233-238.
Dean, B. (1895) The early development of gar-pike and sturgeon. J. Morphol. 11, 1-53.
De Jesus, E.G., Hirano, T. and Inui, Y. (1991) Changes in cortisol and thyroid hormone concentrations during early development and metamorphosis in the Japanese flounder, Paralichthys olivaceus. Gen. Comp. Endocrin. 82, 369-376.
Echelle, A.A. and Riggs, C.D. (1972) Aspects of the early life history of gars (Lepisosteus) in Lake Texoma. Transactions of the American Fisheries Society 101, 106-112.
Erlanger, F.E., Kokowsky, N. and Cohen, W. (1961) The preparation and properties of two new chromogenic substrates of trypsin. Archs. Biochem. Biophys. 95, 271-278.
Ferran, A.M. (1996). Análisis discriminante. In: McGraw Hill (ed.), SPSS para Windows, Programación y análisis estadístico, pp. 287-312.
Galgani, F. and Nagayama, F. (1986) Characteristic of digestive proteolysis of the crabs Portunus trituberculatus, Portunus sanguinolentus and Charybdis japonica. Bull. Japan. Soc. Sci. Fish. 52, 2183-2188.
Garcia-Carreño, F.L., Dimes, L.E. and Haard, N.F. (1993) Substrate-gel electrophoresis for composition and molecular weight of proteinases or proteinaceus proteinase inhibitors. Analyt. Biochem. 214, 65-69.
Gawlicka, A., The, S.J., Hung, S.S., Hinton, D.E. and De la Noue, J. (1995) Histological and histochemical changes in the digestive tract of white sturgeon larvae during ontogeny. Fish Physiol. Biochem. 14, 357-371.
Kobuke, L., Specker, J. and Berni, H.A. (1987) Thyroxine content of eggs and larvae of coho salmon Oncorhynchus kisutch. J. Exp. Zool. 248, 168-176.
Lauf, M. and Hoffer, R. (1984) Proteolytic enzymes in fish development and the importance of dietary enzymes. Aquaculture 37, 335-346.
May, E.B. and Echelle, A. (1968). Young-of-year alligator gar in Lake Texoma, Oklahoma. Copeia 3, 629-630.
Morales, G. (1987) Reproducción y desarrollo embriológico del catán (Lepisosteus spatula Lacepede). Primeros resultados. In: Secretaría de Pesca (ed.), Manual Técnico para el aprovechamiento de Existencias Silvestres, pp. 41-70.
Moore, G., Trautman, M. and Curd, M. (1973) Description of postlarval gar (Lepisosteus spatula Lacepede, Lepisosteidae), with a list of associated species from the Red River, Choctaw County, Oklahoma. The Southwest Naturalist 18, 343-344.
Moyano, F.J., Diaz, M., Alarcon, F.J. and Sarasquete, M.C. (1996) Characterization of digestive enzyme activity during larval development of gilthead seabream (Sparus aurata). Fish Physiol. Biochem. 15, 121-130.
Munilla-Moran, R. and Saborido, F. (1996) Digestive enzymes in marine species I. Proteinases activities in gut from red fish (Sebastes mentella), seabream (Sparus aurata) and turbot (Scophtalmus maximus). Comp. Biochem. Physiol. 113B, 395-402.
Person-Le Rouyet, J. (1990) Early weaning of fish larvae onto microdiets: Constraints and perspectives. In: Advances in Tropical Aquaculture. Aquacop-IFREMER, Actes de Colloque 9, pp. 625-642.
SEPESCA-INP (1994) Atlas pesquero de México. In: Rodriguez De La Cruz, M.C., Palacios-Fest, M.R., Cruz-Santabalbina, R. y Dias (eds.), INP/Subsecretaria de Pesca, México, pp. 182-183.
Simon, T.P. and Wallus, R. (1989) Contributions to the early life histories of gar (Actynopterygii: Lepisosteidae) in the Ohio and 142 Tennessee river basins with emphasis on larval development. Trans. Ky. Acad. Sci. 50, 59-74.
Simon, T.P. and Tyberghein, E.J. (1991) Contributions to the early life history of the spotted gar, Lepisosteus oculatus Winchell, from Hatchet Creek, Alabama. Trans. Ky. Acad. Sci. 52(3-4), 124-131.
Suttkus, R.D. (1963) Order lepisostei. Fishes of the Western North Atlantic. Mem. Sears Found. Mar. Res. 1(3), 61-88.
Theilacker, G.H and Porter, S.M. (1994) Condition of larval walleye pollock, Theragra chalcogramma, in the western Gulf of Alaska assessed with histological and shrinkage indices. Fish. Bull. 93, 333-344.
Vonk, H.J. and Western, J.R. (1984) Proteinases of sub-mammalian vertebrates. In: Academic Press (ed.), Comparative Biochemistry and Physiology of Enzymatic Digestion, pp. 135-183.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mendoza, R., Aguilera, C., Rodríguez, G. et al. Morphophysiological studies on alligator gar (Atractosteus spatula) larval development as a basis for their culture and repopulation of their natural habitats. Reviews in Fish Biology and Fisheries 12, 133–142 (2002). https://doi.org/10.1023/A:1025047914814
Issue Date:
DOI: https://doi.org/10.1023/A:1025047914814