Abstract
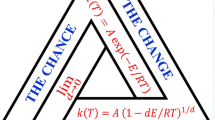
Traditional phenomenological kinetics describes the process rate in terms of the progress degree (mass fraction which has already undergone the change), a, and with a monomial function (or combination of monomial functions for multistep processes) of (1-a), without any connection to the underlying mechanism at the molecular level. The approach proposed in the present work aims at the direct treatment of the experimental data, like DSC records, without suggesting any specific reaction mechanism and excluding any Arrhenius like behaviour. Formal expressions are proposed that include the thermodynamic constraints for any spontaneous process, viz. a negative drop of the Gibbs function throughout the process, and describe the process rate as the result of the effects of a thermodynamic driving force, identified with the drop of the Gibbs function, and of the medium hindrance.
Similar content being viewed by others
References
H. Eyring and E. M. Eyring, Modern Chemical Kinetics, Van Nostrand Reinhold, London 1963.
F. Lindemann, see discussion reported in K. J. Laidler, Chemical Kinetics, Harper and Row Publ., 1987.
E. Santacesaria, Catalysis Today, 52 (1999) 113.
S. Wang and H. Hofmann, Chem. Eng. Sci., 54 (1999) 1639.
S. Toxvaerd, Comp. Phys. Comm., 121–122 (1999) 251.
J. Sempere, R. Nomen and R. Serra, J. Therm. Anal. Cal., 56 (1999) 843 and therein quoted previous works by the same Authors.
J. Šesták and Z. Chvoj, 2001, private communication to be submitted for publication.
G. Nicolis, Rep. Progr. Phys., 42 (1979) 225.
D. K. Kondepudi and I. Prigogine, Modern Thermodynamics: from Heat Engines to Dissipative Processes, Wiley, London 1998.
A. Schiraldi, L. Piazza, D. Fessas and M. Riva, in Handbook of Thermal Analysis and Calorimetry, Vol. 4, From Macromolecules to Man, R. B. Kemp Ed., Elsevier Publ., Amsterdam 1999, pp. 829-921, see appendix of the paper.
M. Riva, A. Schiraldi and L. Piazza, Thermochim. Acta, 246 (1994) 317.
M. Riva and A. Schiraldi, Thermochim. Acta, 220 (1993) 117.
D. Fessas and A. Schiraldi, J. Therm. Anal. Cal., 61 (2000) 411.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schiraldi, A. Phenomenological Kineticsan; an alternative approach. Journal of Thermal Analysis and Calorimetry 72, 885–900 (2003). https://doi.org/10.1023/A:1025026517253
Issue Date:
DOI: https://doi.org/10.1023/A:1025026517253