Abstract
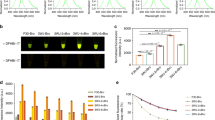
Double-stranded RNA interference technology has recently been shown to be a powerful tool to silence gene expression in various organisms, including plants. Sustained double-stranded RNA interference mediated gene silencing is normally triggered by hairpin RNAs generated by in vivo transcription of inverted repeat DNA constructs. To test the efficiency of inverted repeat constructs for their in vivo gene silencing capability, we developed an assay that is based on transient expression in single cells using two fluorescent reporter proteins with different emission spectra. We co-expressed one fluorescent protein as marker for transfected cells and the second as translational fusion with the target gene. Co-transfection of a vector mediating expression of inverted repeats of the target gene resulted in a specific decrease or undetectable fusion protein fluorescence in the majority of transfected cells.
Similar content being viewed by others
References
Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE & Mello CC (1998) Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391: 806–811.
Nishikura K (2001) A short primer on RNAi: RNA-directed RNA ploymerase acts as a key catalyst. Cell 107: 415–418.
Kennderell J & Carthew R (1998) Drosophila frizzled and frizzled2 act in the wingless pathway as determined by dsRNA-mediated genetic interference. Cell 95: 1017–1026.
Ngo H, Tschudi C, Gull K & Ullu E (1998). Double-stranded RNA induces mRNA degradation in Trypanosoma brucei. Proc Natl Acad Sci. 95: 14687–14692.
Wargelius A, Ellingsen S & Fjose A (1999) Double-stranded RNA induces specific developmental defects in zebrafish embryos. Biochem Biophys Res Commun 263: 156–161.
Chuang CF & Meyerowitz EM (2000) Specific and heritable genetic interference by double-stranded RNA in Arabidopsis thaliana. Proc Natl Acad Sci 97: 4985–4990.
Schweizer P, Pokorny J, Schulze-Lefert P & Dudler R (2000) Double-stranded RNA interferes with gene function at the single-cell level in cereals. Plant J 24: 895–903.
Tavernarakis N, Wang SL, Dorovkov M, Ryazanov A & Driscoll M (2000) Heritable and inducible genetic interference by double-stranded RNA encoded by transgenes. Nature Genetics 24: 180–183.
Horton RM, Hunt HD, Ho SN, Pullen JK & Pease LR (1989) Engineering hybrid genes without the use of restriction enzymes - gene splicing by overlap extension. Gene 77: 61–68.
Ling V & Zielinski RE (1989) Cloning of cDNA sequences encoding the calcium-binding protein, calmodulin, from barley (Hordeum vulgare L.). Plant Physiol 90: 714–719.
Jach G, Binot E, Frings S, Luxa K & Schell J (2001) Use of red fluorescent protein from Discosoma sp. (dsRED) as a reporter for plant gene expression. Plant J 28: 483–491.
Zhou FS, Kurth J, Wei FS, Elliott C, Vale G, Yahiaoui N, Keller B, Somerville S, Wise R & Schulze-Lefert P (2001) Cell-autonomous expression of barley Mla1 confers race-specific resistance to the powdery mildew fungus via a Rar1-independent signaling pathway. Plant Cell 13: 337–350.
Shirasu K, Nielsen K, Piffanelli P, Oliver R & Schulze-Lefert P (1999) Cell-autonomous complementation of mlo resistance using a biolistic transient expression system. Plant J 17: 293–299.
Smith NA, Singh SP, Wang MB, Stoutjesdijk PA, Green AG & Waterhouse PM (2000) Gene expression - Total silencing by intron-spliced hairpin RNAs. Nature 407: 319–320.
Kim MC, Panstruga R, Elliott C, Müller J, Devoto A, Yoon HW, Park HC, Cho MJ & Schulze-Lefert P (2002) Calmodulin interacts with MLO protein to regulate defence against mildew in barley. Nature 416: 447–450.
Azevedo C, Sadanandom A, Kitagawa K, Freialdenhoven A, Shirasu K & Schulze-Lefert P (2002) The RAR1 interactor SGT1 is an essential component of R-gene triggered disease resistance. Science 295: 2073–2076.
Yang D, Hong L & Erickson JW (2000) Evidence that processed small dsRNAs may mediate sequence-specific mRNA degradation during RNAi in Drosophila embryos. Curr Biol 10: 1191–1200.
Sijen, T, Fleenor J, Simmer F, Thijssen KL, Parrish S, Timmons L, Plasterk RHA & Fire A (2001) On the role of RNA amplification in dsRNA-triggered gene silencing. Cell 107: 465–476.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Panstruga, R., Chul Kim, M., Cho, M.J. et al. Testing the efficiency of dsRNAi constructs in vivo: A transient expression assay based on two fluorescent proteins. Mol Biol Rep 30, 135–140 (2003). https://doi.org/10.1023/A:1024945920331
Issue Date:
DOI: https://doi.org/10.1023/A:1024945920331