Abstract
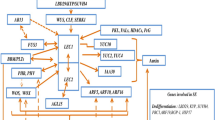
The main approaches have been considered to studying the genetic control of plant cell totipotency in an in vitro culture. The capacity of cultured plants for callusogenesis, organ formation, and somatic embryogenesis depends on the activity of genes that determine and maintain the meristematic state of cells, level of hormones in the cells, and sensitivity to hormones, as well as on the activity other genes that control different stages of plant morphogenesis.
Similar content being viewed by others
References
Agache, S., Bachelier, B., De Buyser, J., et al., Genetic Analysis of Anther Culture Response in Wheat Using Aneuploid, Chromosome Substitution, and Translocation Lines, Theor. Appl. Genet., 1989, vol. 77, pp. 7-11.
Aida, M., Ishida, T., and Tasaka, M., Shoot Apical Meristem and Cotyledon Formation during Arabidopsis Embryogenesis: Interaction Among the CUP-SHAPED COTYLEDON and SHOOT MERISTEMLESS Genes, Development (Cambridge, UK), 1999, vol. 126, pp. 1563-1570.
Armstrong, C.L., Romero-Severson, J., and Hodges, T.K., Improved Tissue Culture Response of an Elite Maize Inbred through Backcross Breeding, and Identification of Chromosomal Regions Important for Regeneration by RFLP Analysis, Theor. Appl. Genet., 1992, vol. 84, pp. 755-762.
Avetisov, V.A. and Melik-Sarkisov, O.S., Genotypic Features of Morphogenesis in Callus Cultures of Various Potato Cultivars, S.-Kh. Biol., 1985, no. 3, pp. 67-70.
Barton, M.K. and Poethig, R.S., Formation of the Shoot Apical Meristem in Arabidopsis thaliana: An Analysis of Development in the Wild Type and shoot meristemless Mutant, Development (Cambridge, UK), 1993, vol. 119, pp. 823-831.
Beaumont, V.H., Rocheford, T.R., and Widholm, J.M., Mapping the Anther Culture Response Genes in Maize (Zea mays L.), Genome, 1995, vol. 38, pp. 968-975.
Benjamins, R., Quint, A., Weijers, D., et al., The PINOID Protein Kinase Regulates Organ Development in Arabidopsis by Enhancing Polar Auxin Transport, Development (Cambridge, UK), 2001, vol. 128, pp. 4057-4067.
Bentolila, S., Hardy, T., Guitton, C., et al., Comparative Genetic Analyses of F2 Plants and Anther Culture Derived Plants of Maize, Genome, 1992, vol. 35, pp. 575-582.
Boerjan, W., Cervera, M.T., Delarue, M., et al., superroot, a Recessive Mutation in Arabidopsis, Confers Auxin Overproduction, Plant Cell, 1995, vol. 7, pp. 1405-1419.
Breton, A.M. and Sung, Z.R., Temperature-Sensitive Carrot Variants Impaired in Somatic Embryogenesis, Dev. Biol., 1982, vol. 90, pp. 58-66.
Butenko, R.G., Eksperimental'nyi morfogenez i differentsiatsiya v kul'ture kletok rastenii (Experimental Morphogenesis and Differentiation in Culture of Plant Cells), Moscow: Nauka, 1975.
Buzovkina, I.S., Lutova, L.A., and Kneshke, I., Genetic Analysis of the Feature “Sensitivity to Cytokinin” in Radish in vitro, Genetika, 1993, vol. 29, no. 6, pp. 995-1001.
Cary, A.J., Uttamchandani, S.J., Smets, R., et al., Arabidopsis Mutants with Increased Organ Regeneration in Tissue Culture Are More Competent to Respond to Hormonal Signals, Planta, 2001, vol. 213, no. 5, pp. 700-707.
Cary, A.J., Che, P., and Howell, S.H., Developmental Events and Shoot Apical Meristem Gene Expression Patterns during Shoot Development in Arabidopsis thaliana, Plant J., 2002, vol. 32, no. 6, pp. 867-877.
Che, P., Gingerich, D.J., Lall, S., and Howell, S.H., Global and Hormone-Induced Gene Expression Changes during Shoot Development in Arabidopsis, Plant Cell, 2002, vol. 14, no. 11, pp. 2771-2785.
Chernysheva, V.G. and Shamina, Z.B., Influence of TsMS on Induction of Embryogenic Callus Culture of Maize, Genetika, 1990, vol. 26, no. 8, pp. 1436-1439.
Chernysheva, V.G., Dolgikh, Yu.I., Shamina, Z.B., and Butenko, R.G., Influence of Genetic Characteristics on Morphogenetic Potential of Callus Maize Cells, Dokl. Akad. Nauk SSSR, 1988, vol. 300, no. 1, pp. 227-229.
Christensen, S.K., Dagenais, N., Chory, J., and Weigel, D., Regulation of Auxin Response by the Protein Kinase PINOID, Cell (Cambridge, Mass.), 2000, vol. 100, pp. 469-478.
Chuck, G., Lincoln, C., and Hake, S., KNAT1 Induces Lobed Leaves with Ectopic Meristems When Overexpressed in Arabidopsis, Plant Cell, 1996, vol. 8, pp. 1277-1289.
Clark, S.E., Running, M.P., and Meyerowitz, E.M., CLAVATA3 Is a Specific Regulator of Shoot and Floral Meristem Development Affecting the Same Processes as CLAVATA1, Development (Cambridge, UK), 1995, vol. 121, pp. 2057-2067.
Clark, S.E., Jacobsen, S.E., Levin, J., and Meyerowitz, E.M., The CLAVATA and SHOOT MERISTEMLESS Loci Competitively Regulate Meristem Activity in Arabidopsis, Development (Cambridge, UK), 1996, vol. 122, pp. 1567-1575.
Coleman, M., Waugh, R., and Powell, W., Genetic Analysis of in vitro Culture Response in Potato, Plant Cell Tissue Organ Culture, 1990, vol. 23, no. 3, pp. 181-186.
Cowen, N.M., Johnson, C.D., Armstrong, K., et al., Mapping Genes Conditioning in vitro Androgenesis in Maize Using RFLP Analysis, Theor. Appl. Genet., 1992, vol. 84, pp. 720-724.
De Jong, A.J., Hendriks, T., Meijer, E., et al., Transient Reduction in Secreted 32 KD Chitinase Prevents Somatic Embryogenesis in the Carrot (Daucus carota L.) Variant Ts11, Devel. Genet., 1995, vol. 16, pp. 332-343.
Delarue, M., Prinsen, E., Van Onckelen, H., et al., Sur2 Mutations of Arabidopsis thaliana Define a New Locus Involved in the Control of Auxin Homeostasis, Plant J., 1998, vol. 14, pp. 603-611.
Endrizzi, K., Moussian, B., Haecker, A., et al., The SHOOT MERISTEMLESS Gene Is Required for Maintenance of Undifferentiated Cells in Arabidopsis Shoot and Floral Meristems and Acts at a Different Regulatory Level than the Meristem Genes WUSCHEL and ZWILLE, Plant J., 1996, vol. 10, pp. 101-113.
Ezhova, T.A. and Kovalenko, O.V., Manifestation of the Lf Locus in Tissue Culture of Pea, Pisum Genet., 1992, vol. 24, pp. 40-43.
Ezhova, T.A., Bagrova, A.M., and Gostimskii, S.A., Shoot Formation in Calluses from Stem Apices, Epicotyls, and Leaf Inernodes of Various Pea Genotypes, Fiziol. Rast. (Moscow), 1985, vol. 32, no. 3, pp. 513-520.
Ezhova, T.A., Bagrova, A.M., and Gostimski, S.A., Cell Selection as a Possible Reason for the Specificity of Somaclonal Variation in Pea, Plant Breeding, 1995, vol. 114, pp. 520-524.
Ezhova, T.A., Soldatova, O.P., and Kof, E.M., Involvement of Gene ABRUPTUS Controlling Morphogenesis of Arabidopsis thaliana in Regulation of Growth and Morphogenesis in an in vitro Culture, Fiziol. Rast. (Moscow), 1999, vol. 46, no. 6, pp. 865-870.
Ezhova, T.A., Soldatova, O.P., Kalinina, A.Yu., et al., Interactions of Genes ABRUPTUS/PINOID and LEAFY during Floral Morphogenesis in Arabidopsis thaliana (L.) Heynh., Genetika, 2000, vol. 36, no. 12, pp. 1-6.
Fadeeva, T.S., Lutova, L.A., and Kozyreva, O.G., A Study of Plant Regenration as a Genetic Character Using the Method of Cultire of Isolated Organs, Issledovaniya po genetike (Studies on Genetics), Leningrad: Leningrad. Gos. Univ., 1974, no. 5, pp. 63-71.
Fadeeva, T.S., Lutova, L.A., and Kozyreva, O.G., Plan Regeneration as A Genetic Character, Issledovaniya po genetike (Studies on Genetics), Leningrad: Leningrad. Gos. Univ., 1979, no. 8, pp. 160-170.
Felsenburg, T., Feldman, M., and Galun, E., Aneuploid and Alloplasmin Lines as Tools for the Study of Nuclear and Cytoplasmic Control of Culture Ability and Regeneration of Scutellar Calli from Common Wheat, Theor. Appl. Genet., 1987, vol. 74, pp. 802-810.
Frank, M., Guivarc'h, A., Krupkova, E., et al., Tumorous Shoot Development (TSD) Genes Are Required for Co-Ordinated Plant Shoot Development, Plant J., 2002, vol. 29, no.?1, pp. 73-85.
Frank, M., Rupp, H.-M., Prinsen, E., et al., Hormone Autotrophic Growth and Differentiation Identifies Mutant Lines of Arabidopsis with Altered Cytokinin and Auxin Content or Signaling, Plant Physiol., 2000, vol. 122, pp. 721-730.
Galiba, G., Kovácz, G., and Sutka, J., Substitution Analysis of Plant Regeneration from Callus Culture in Wheat, Plant Breed., 1986, vol. 97, pp. 261-263.
Giuliano, G., Lo Schiavo, F., and Terzi, M., Isolation and Developmental Characterization of Temperature-Sensitive Carrot Cell Variants, Theor. Appl. Genet., 1984, vol. 67, pp. 179-183.
Hayashi, H., Czaja, I., Lubenow, H., et al., Activation of a Plant Gene by T-DNA Tagging: Auxin-Independent Growth in vitro, Science, 1992, vol. 258, pp. 1350-1353.
Hecht, V., Vielle-Calzada, J.-P., Hartog, M.V., et al., The Arabidopsis Somatic Embryogenesis Receptor Kinase 1 Gene Is Expressed in Developing Ovules and Embryos and Enhances Embryogenic Competence in Culture, Plant Physiol., 2001, vol. 127, pp. 803-816.
Helleboid, S., Hendriks, T., Bauw, G., et al., Three Major Somatic Embryogenesis Related Proteins in Cichorium Identified as PR Proteins, J. Exp. Bot., 2000, vol. 51, no. 348, pp. 1189-1200.
Henry, Y. and De Buyser, J., Effect of the 1B/1R Translocation on Anther Culture Ability in Wheat (Triticum aestivum L.), Plant Cell, 1985, vol. 4, pp. 307-310.
Henry, Y., Vain, P., and De Buyser, J., Genetic Analysis of in vitro Plant Tissue Culture Responses and Regeneration Capacities, Euphytica, 1994a, vol. 79, pp. 45-58.
Henry, Y., Marcotte, J.-L., and De Buyser, J., Chromosomal Location of Genes Controlling Short-Term and Long-Term Somatic Embryogenesis in Wheat Revealed by Immature Embryo Culture of Aneuploid Lines, Theor. Appl. Genet., 1994b, vol. 89, pp. 344-350.
Isaeva, N.A., Shumnyi, V.K., and Pershina, L.A., A Study of Specific Features of Callusogenesis in Different Barley Species, Izv. Sib. Otd. Akad. Nauk SSSR, Ser. Biol. Nauki, 1980, no. 1, pp. 7-74.
Kakimoto, T., CKI1, a Histidine Kinase Homolog Implicated in Cytokinin Signal Transduction, Science, 1996, vol. 274, pp. 982-985.
Kakimoto, T., Genes Involved in Cytokinin Signal Transduction, J. Plant Res., 1998, vol. 111, pp. 261-265.
King, J.J., Stimart, D.P., Fisher, R.H., and Bleecker, A.B., A Mutation Altering Auxin Homeostasis and Plant Morphology in Arabidopsis, Plant Cell, 1995, vol. 7, pp. 2023-2037.
Komatsuda, T., Taguchi-Shiobara, F.F., Oka, S., et al., Transfer and Mapping of the Shoot-Differentiation Locus Shd1 in Barley Chromosome 2, Genome, 1995, vol. 38, pp. 1009-1014.
Koornneef, M., Bade, J., Hanhart, C., et al., Characterization and Mapping of a Gene Controlling Shoot Regeneration in Tomato, Plant J., 1993, vol. 3, pp. 131-141.
Kubo, M. and Kakimoto, T., The Cytokinin-Hypersensitive Genes of Arabidopsis Negatively Regulate the Cytokinin-Signaling Pathway for Cell Division and Chloroplast Development, Plant J., 2000, vol. 23, no. 3, pp. 385-394.
Kucherenko, L.A. and Mamaeva, G.G., Callusogenesis and Organogenesis in Tissue Culture of Various Rice Cultivars, S.-Kh. Biol., 1980, no. 3, pp. 384-386.
Kwon, Y.S., Kim, K.M., Eun, M.Y., and Sohn, J.K., Quantitative Trait Loci Mapping Associated with Plant Regeneration Ability from Seed Derived Calli in Rice (Oryza sativa L.), Mol. Cells, 2001, vol. 28, no. 11(1), pp. 64-67.
Lincoln, C., Long, J., Yamaguchi, J., et al., Knotted1-Like Homeobox Gene in Arabidopsis Is Expressed in the Vegetative Meristem and Dramatically Alters Leaf Morphology when Overexpressed in Transgenic Plants, Plant Cell, 1994, vol. 6, pp. 1859-1876.
Lo Schiavo, F., Giuliano, G., and Sung, Z.R., Characterization of Temperature-Sensitive Carrot Cell Mutant Impaired in Somatic Embryogenesis, Plant Sci. (Limerick, Irel.), 1988, vol. 54, pp. 157-164.
Long, J.A., Moan, E.I., Medford, J.I., and Barton, M.K., A Member of the KNOTTED Class of Homeodomain Proteins Encoded by the STM Gene of Arabidopsis, Nature (London), 1996, vol. 379, pp. 66-69.
Lotan, T., Ohto, M., Yee, K.M., et al., Arabidopsis LEAFY COTYLEDON1 Is Sufficient to Induce Embryo Development in Vegetative Tissue, Cell (Cambridge, Mass.), 1998, vol. 93, pp. 1195-1205.
Lund, S.T., Smith, A.G., and Hackett, W.P., Cuttings of a Tobacco Mutant, rac, Undergo Cell Divisions but Do Not Initiate Adventitious Roots in Response to Exogenous Auxin, Physiol. Plant., 1996, vol. 97, pp. 372-380.
Lund, S.T., Smith, A.G., and Hackett, W.P., Differential Gene Expression in Response to Auxin Treatment in the Wild Type and rac, an Adventitious Rooting-Incompetent Mutant of Tobacco, Plant. Physiol., 1997, vol. 114, pp. 1197-1206.
Lutova, L.A. and Kozyreva, O.G., Genetics of Morphogenesis in Plant Tissue Culture, Issledovaniya po genetike (Studies on Genetics), Leningrad: Leningrad. Gos. Univ., 1985, vol. 10, pp. 113-118.
Mano, Y., Takahashi, H., Sato, K., and Takeda, K., Mapping Genes for Callus Growth and Shoot Regeneration in Barley (Hordeum vulgare L.), Breed. Sci., 1996, vol. 46, pp. 137-142.
Mathias, R.J. and Atkinson, E., in vitro Expression of Genes Affecting Whole Plant Phenotype—the Effect of Rht/Gai Alleles on the Callus Culture Response of Wheat (Triticum aestivum L. em Thell), Theor. Appl. Genet., 1988, vol. 75, pp. 474-479.
Mayer, K.F., Schoof, H., Haecker, A., et al., Role of WUSCHEL in Regulating Stem Cell Fate in the Arabidopsis Shoot Meristem, Cell (Cambridge, Mass.), 1998, vol. 95, no. 6, pp. 805-815.
Mordhorst, A.P., Voerman, K.J., Hartog, M.V., et al., Somatic Embryogenesis in Arabidopsis thaliana Is Facilitated by Mutations in Genes Repressing Meristematic Cell Divisions, Genetics, 1998, vol. 149, pp. 549-563.
Muller, J.-F., Goujaud, J., and Caboche, M., Isolation in vitro of Naphthaleneacetic Acid-Tolerant Mutants of Nicotiana tabacum, Which Are Impaired in Root Morphogenesis, Mol. Gen. Genet., 1985, vol. 199, pp. 194-200.
Murashko, L.N. and Fadeeva, T.S., A Study of the Pattern of Regeneration in Different Pea Forms, Vestn. Leningr. Univ., 1973, vol. 3, no. 15, p. 132.
Ogas, J., Cheng, J.-C., Sung, Z.R., and Somerville, C., Cellular Differentiation Regulated by Gibberellin in the Arabidopsis thaliana Pickle Mutant, Science, 1997, vol. 277, no.?5322, pp. 91-94.
Ogas, J., Kaufmann, S., Henderson, J., and Somerville, C., PICKLE Is a CHD3 Chromatin-Remodeling Factor That Regulates the Transition from Embryogenic to Vegetative Development in Arabidopsis, Proc. Natl. Acad. Sci. USA, 1999, vol. 96, pp. 13 839-13 844.
Omel'yanchuk, N.A. and Gvozdev, A.V., Use of Isogenic Lines for Studies of Callusogenesis and Regeneration, Ispol'zovanie izogennykh linii v selektsionno-geneticheskikh eksperimentakh (Use of Isogenic Lines in Selection-Genetic Experiments), Novosibirsk, 1990, pp. 96-98.
Rupp, H.M., Frank, M., Werner, T., et al., Increased Steady State MRNA Levels of the STM and KNAT1 Homeobox Genes in Cytokinin Overproducing Arabidopsis thaliana Indicate a Role for Cytokinins in the Shoot Apical Meristem, Plant J., 1999, vol. 18, no. 5, pp. 557-563.
Schmidt, E.D.L., Guzzo, F., Toonen, M.A.J., and de Vries, S.C., A Leucine-Rich Repeat Containing Receptorlike Kinase Marks Somatic Plant Cells Competent to Form Embryos, Development (Cambridge, UK), 1997, vol. 124, pp. 2049-2062.
Schnall, J.A., Cooke, T.J., and Cress, D.E., Genetics Analysis of Somatic Embryogenesis in Carrot Cell Culture: Initial Characterization of Six Classes of Temperature-Sensitive Variants, Devel. Genet., 1988, vol. 9, pp. 49-67.
Schoof, H., Lenhard, M., Haecker, A., et al., The Stem Cell Population of Arabidopsis Shoot Meristems Is Maintained by a Regulatory Loop between the CLAVATA and WUSCHEL Genes, Cell (Cambridge, Mass.), vol. 100, pp. 635-644.
Seo, M., Akaba, S., Oritani, T., et al., Higher Activity of an Aldehyde Oxidase in the Auxin-Overproducing superroot1 Mutant of Arabidopsis thaliana, Plant Physiol., 1998, vol. 116, pp. 687-693.
Sugiyama, M., Organogenesis in vitro, Curr. Opin. Plant Biol., 1999, vol. 2, pp. 61-64.
Taguchi-Shiobara, F., Lin, S.Y., Tanno, K., et al., Mapping Quantitative Trait Loci Associated with Regeneration Ability of Seed Callus in Rice, Oriza sativa L, Theor. Appl. Genet., 1997, vol. 95, pp. 828-833.
Tong, J.K., Hassig, C.A., Schnitzler, G.R., et al., Chromatin Deacetylation by an ATP-Dependent Nucleosome Remodelling Complex, Nature (London), 1998, vol. 395, pp. 917-921.
Trotochaud, A.E., Hao, T., Wu, G., et al., The CLAVATA1 Receptor-Like Kinase Requires CLAVATA3 For Its Assembly into a Signaling Complex That Includes KAPP and a Rho-Related Protein, Plant Cell, 1999, vol. 11, pp. 393-405.
Van Hengel, A.J., Guzzo, F., van Kammen, A., and de Vries, S.C., Expression Pattern of the Carrot EP3 Endochitinase Genes in Suspension Cultures and in Developing Seeds, Plant Physiol., 1998, vol. 117, pp. 43-53.
Vysotskii, V.A., Clonal Micropropagation of Plants, Kul'tury kletok rastenii i biotekhnologiya (Cultures of Plant Cells and Biotechnology), Moscow: Nauka, 1986, pp. 91-102.
Weller, J.L., Reid, J.B., Taylor, S.A., and Murfet, I.C., The Genetic Control of Flowering in Pea, Trends Plant Sci., 1997, vol. 2, pp. 412-418.
Yasutani, I., Ozawa, S., Nishida, T., et al., Isolation of Temperature-Sensitive Mutants of Arabidopsis thaliana That Are Defective in the Redifferentiation of Shoots, Plant Physiol., 1994, vol. 105, pp. 815-822.
Zhong, R., Kays, S.J., Schroeder, B.P., and Ye, Z.-H., Mutation of a Chitinase-like Gene Causes Ectopic Deposition of Lignin, Aberrant Cell Shapes, and Overproduction of Ethylene, Plant Cell, vol. 14, no. 1, pp. 165-179.
Zuo, J., Niu, Q.W., Frugis, G., and Chua, N.H., The WUSCHEL Gene Promotes Vegetative-to-Embryonic Transition in Arabidopsis, Plant J., 2002, vol. 30, no. 3, pp. 349-359.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ezhova, T.A. Genetic Control of Totipotency of Plant Cells in an in vitro Culture. Russian Journal of Developmental Biology 34, 197–204 (2003). https://doi.org/10.1023/A:1024940130511
Issue Date:
DOI: https://doi.org/10.1023/A:1024940130511