Abstract
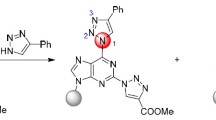
The following paper summarizes our work on compound libraries of 2,6,8-trisubstituted purines. This synthesis route on a polystyrene support begins with 2,6-dichloro purine making extensive use of catalysis. During the synthesis the polymer bound purines were brominated selectively on C8. The substitution reaction of C6-Cl by amines was found to be acid catalyzed. The substitution of C2-Cl by amines and aryls, as well as the substitution of a C8-Br by aryls, alkenyl and alkynyl groups can be catalyzed by transition metals. Under some bromination conditions novel selective oxidative transformations of 2-amino groups in 2,6-diamino purines have been found.
Similar content being viewed by others
References
Denessiouk, K. A., Rantanen, V.-V. and Johnson, M. S., Adenine recognition: a motif present in ATP-, CoA-, NAD-, NADP-, and FAD-dependent proteins, Proteins: Structure, Function, and Genetics, 44 (2001) 282–291.
Denessiouk K. A. and Johnson M. S. When fold is not important: a common structural framework for adenine and AMP binding in 12 unrelated protein families, Proteins: Structure, Function, and Genetics, 38 (2000) 310–326.
For instance: a) Fischer, E. Synthese des Hypoxanthins, Xanthins, Adenins und Guanins, Ber., 30 (1887), 2226–2254.
Fischer, E. and Ach. L. Ueber das Oxydichlorpurin, Ber., 30 (1897) 2208–2219.
Lipinski, C.A., Lombardo, F., Dominy, B.W. and Feeney, P.J., Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings, J. Adv. Drug, Delivery Reviews, 23 (1997) 3–25.
Ertl, P., Rohde, B. and Selzer, P., Fast calculation of molecular polar surface area as a Sum of fragment-based contributions and its application to the prediction of drug transport properties, J. Med. Chem., 43 (2000) 3714–3717.
Walters, W.P. and Murcko, M.A., Library filtering systems and predictions of drug-like properties, Methods Princ. Med. Chem., 10 (2000) 15–32.
Naumann, T. and Matter, H., Structural classification of protein kinases using 3D molecular interaction field analysis of their ligand binding sites: target family landscapes, J. Med. Chem., 45 (2002) 2366–2378.
Gray, N. S., Wodicka, L., Thunnissen, A.-M. W. H., Norman, T. C., Kwon, S., Espinoza, F. H., Morgan, D. O., Barnes, G., LeClerc, S., Meijer, L., Kim, S.-H., Lockhart, D. J. and Schultz, P. G., Exploiting chemical libraries, structure, and genomics in the search for kinase inhibitors, Science (Washington, D. C.), 281 (1998) 533–538.
Schulze-Gahmen, U., Brandsen, J., Jones, H. D., Morgan, D.O., Meijer, L., Vesely, J. and Kim, S.H., Multiple modes of ligand recognition: crystal structures of cyclin-dependent protein kinase 2 in complex with ATP and two inhibitors, olomoucine and isopentenyladenine, Proteins Struct. Funct. Genet., 22 (1995) 378–391.
De Acevedo, W.F., Lecelerc, S., Meijer, L., Havlicek, L., Strnad, M. and Kim, S.H., Inhibition of cyclin-dependent kinases by purine analogs. Crystal structure of human cdk2 complexed with roscovitine, Eur. J. Biochem., 243 (1997) 518–526.
Donohue, J. and Trueblood, K.N., Base pairing in deoxyribonucleic acid, J. Mol. Biol., 2 (1960) 363–371.
Donohue, J., Hydrogen-bonded helical configurations of polynucleotides, Proc. Natl. Acad. Sci. USA., 42 (1956) 60–65.
Chang, Y.-T., Choi, G., Bae, Y.-S., Burdett, M., Moon, H.-S., Lee, J. Wook, Gray, N. S., Schultz, P.G., Meijer, L., Chung, S.-K., Choi, K. Y., Suh, P.-G. and Ryu, S. H., Purine-based inhibitors of inositol-1,4,5-triphosphate-3-kinase, Chem. Bio. Chem., 3 (2002) 897–901.
Verdugo, D.E., Cancilla, M.T., Ge, X., Gray, N.S., Chang, Y.-T., Schultz, P.G., Negishi, M., Leary, J.A. and Bertozzi, C.R., Discovery of estrogen sulfotransferase inhibitors from a purine library screen, J. Med. Chem., 44 (2001) 2683–2686.
Ekstrom, J. L., Pauly, T. A., Carty, M. D., Soeller, W. C., Culp, J., Danley, D. E., Hoover, D. J., Treadway, J. L., Gibbs, E. M., Fletterick, R. J., Day, Y. S. N., Myszka, D. G. and Rath, V. L., Structure-activity analysis of the purine binding site of human liver glycogen phosphorylase, Chem. Biol., (2002) 915–924.
For instance: Norman, T.C., Gray, N.S., Koh, J.T. and Schultz, P.G., Structure-based library approach to kinase inhibitors, J. Am. Chem. Soc., 118 (1996) 7430–7431.
For instance: Makara, G.M., Ewing, W., Ma, Y. and Wintner, E. D., Synthesis of bicyclic pyrimidine derivatives as ATP analogues, J. Org. Chem., 66 (2001) 5783–5789.
Ding, S., Gray, N.S., Wu, X., Ding, Q. and Schultz, P.G., A combinatorial approach toward kinase-directed heterocycle libraries, J. Am. Chem. Soc., 124 (2002) 1594–1596.
Ding, S., Gray, N.S., Ding, Q., Wu, X. and Schultz, P.G., Resin-capture and release strategy toward combinatorial libraries of 2,6,9-substituted purines, J. Comb. Chem., 4 (2002) 183–186.
We used reaction blocks for parallel chemistry in the standard 96-well format obtained from Robbins Scientific Corp., 1250 Elko Drive, Sunnyvale, CA 94089–2213, USA or Charybdis Technologies, Inc. 5925 Priestly Dr. Carlsbad, CA 92008, USA.
For instance: Janeba, Z., Holy, A. and Masojidkova, M., Synthesis of acyclic nucleoside and nucleotide analogs derived from 6-amino-7H-purine-8(9H)-thione and 8-(methylsulfanyl)adenine, Collect. Czech. Chem. Commun. 65, (2000) 1126–1144.
Iwamoto, R.H., Acton, E.M. and Goodman, L., 2'-Deoxythioguanosine and related nucleosides, J.Med. Chem., 6 (1963) 684–688.
Rink, H., Solid-phase synthesis of protected peptide fragments using a trialkoxy-diphenyl-methylester resin, Tetrahedon Lett., 28 (1987) 3787–3790.
Brill, W.K.-D., Schmidt, E. and Tommasi, R.A., Immobilizations of nucleophiles on polystyrene supports, Synlett, (1998) 906–908.
Brill, W.K.-D. Brill, Riva-Toniolo, C. and Mueller, S., Catalysis of 2-and 6-substitution reactions of purines on solid phase, Synlett, 7 (2001) 1097–1100.
Cobb, J.M., Fiorini, M.T., Goddard, C.R., Theoclitou, M.-E. and Abell, C., A decarboxylative traceless linker approach for the solid phase synthesis of quinazolines, Tetrahedron Lett., 40 (1999) 1045–1048.
Parrot, I., Wermuth, C.-G. and Hibert, M., Resin-bound thiophenols as SNAR-labile linkers: Application to the solid phase synthesis of aminopyridazines, Tetrahedron Lett., 40 (1999) 7975–7978.
Adams, R. R. and Whitmore, F. C., Heterocyclic basic compounds. VI. Dialkylamino-alkylaminopurines, J. Am., Chem. Soc., 67 (1945) 1271–1273.
Montgomery, J. A. and Holum, L. B., Synthesis of potential anticancer agents. III. Hydrazino analogs of biologically active purines, J. Am. Chem. Soc., 79 (1957) 2185–2188.
Montgomery, J. A. and Holum, L. B., Synthesis of potential anticancer agents. XI. N2,6-Alkyl derivatives of 2,6-diaminopurine, J. Am. Chem. Soc., 80 (1958) 404–408.
Bresheares, S. R., Wang, S. S., Bechtold, S. G. and Christensen, B. E., Purines. VIII. Aminolysis of chlorosubstituted purines, J. Am. Chem. Soc., 81 (1959) 3789–3791.
Ovcharova, I. M. and Golovchinskaya, E. S., Syntheses of purines. VII. Some transformations of 2,6-dichloro-9-methylpurine, Zh. Obshch. Khim., 34 (1964) 3247–3254.
A substitution at C2 prior to substitution at C6 was ruled out: a) Past literature as in loc.cit 28–32 did not state any observation of those compounds in similar displacements involving 2,6-dichloropurine.
Comparison of UV spectra obtained by HPLC with diode array detection with those of similar compounds in the literature, complemented by MS and 1H-NMR-characterization did and not reveal 6-chloro-2-amino-purines. Relevant literature: loc. cit. 29, 30, and Kwiatkowski, J. S., Electronic structure and spectra of organic molecules. IX. Self-consistent field molecular orbitals configuration interaction calculations for 2,6-disubstituted purines, Theoretica Chimica Acta, 13 (1969) 149–154.
The substitution of the 6-Cl function of 8a-c, 9a, 9b proved to be performable under relatively mild conditions. d) 1H-NMR and UV-spectral analysis of oxidative degradation products 14a, b, d, g, 16 and comparison with the corresponding starting materials supported assumptions on the location of substituents.
Initial NMR, UV and IR references are: a) Coburn, Jr, W. C., Thorpe, M. C., Montgomery, J. A., Hewson, K., Correlation of the proton magnetic resonance chemical shifts of substituted purines with reactivity parameters. I. 2,6-Disubstituted purines, J. Org. Chem., 30 (1965) 1110–1113.
Coburn, Jr, W. C., Thorpe, M. C., Montgomery, J. A. and Hewson, K., Correlation of the proton magnetic resonance chemical shifts of substituted purines with reactivity parameters. II. 6-Substituted purines, J. Org. Chem., 30 (1965) 1114–1117.
Brill, W.K.-D. and Riva-Toniolo, C., The bromination of purines with a charge transfer complex between bromine and lutidine, Tetrahedron Lett., 42 (2002) 6279–6382.
Reddy, N.P. and Tanaka, M., Palladium catalyzed amination of aryl chlorides, Tetrahedron Lett., 38 (1997) 4807–4810.
Hartwig, J.F., Kawatsura, M., Hauck, S.I., Shaughnessy, K.H. and Alcazar-Roman, L.M. Roman, Luis M., Roomtemperature palladium-catalyzed amination of aryl bromides and chlorides and extended scope of aromatic C-N bond formation with a commercial ligand, J. Org. Chem., 64 (1999) 5575–5580.
Irori KansTM: Discovery Partners International, 9640 Towne Centre Drive San Diego, CA 92121, USA.
Miyaura, N., Yanagi, T. and Suzuki, A., Synth. Commu., 11 (1981) 513–519.
For instance: Nolsoe, J.M.J., Gundersen, L.-L. and Rise, F., The palladium-catalyzed cross-coupling reaction of phenylboronic acid with haloarenes in the presence of bases, Synth. Commun., 28, (1998) 4303–4315.
Bruns, G., Ueber Adenine und Hypoxanthin, Ber. Dtsch. Chem. Ges., 23 (1890) 225–229.
Fischer, E. and Reese, L., Liebigs Annalen Chem., 221 (1883) 336–344.
Mishra, M.K., Lenka, S. and Nayak, P.L., Photopolymerization initiated by charge-transfer complex. I. Photopolymerization of methyl methacrylate with the use of quinaldinebromine and lutidine-bromine charge-transfer complexes as photoinitiator, J. Polym. Sci., Polym. Chem. Ed., 19 (1981) 2457–2464.
Hantsch, A., Ueber die vermeintlichen Imid-und Amidchloride, die Salze der Nitrile und Saeureamide, sowie ueber den Chemismus der Umwandlung von Nitrilen in Saeureamide, Ber., 64 (1931) 667–678.
Janz, G.J. and Danyluk, S.S., Conductances of hydrogen halides in anhydrous polar organic solvents, J. Chem. Rev., 60 (1960) 209–234.
Ta-Shma, R. and Rappoport, Z., Nucleophilic attacks on carbon-nitrogen double bonds. Diversity of mechanisms for the substitution of diarylimidoyl chlorides by amines in benzene, J. Am. Chem. Soc., 99 (1977) 1845–1858.
Ugi, I., Beck, F. and Fetzer, U., Hydrolyse von Carbonsaeureimidochloriden, Chem Ber., 95 (1962) 126–135.
Wendeborn, S., De Mesmaeker, A., Brill, W.K.-D., Berteina, S., Synthesis of diverse and complex molecules on the solid phase, Acc. Chem. Res., 33 (2000) 215–224.
Ozola, V., Persson, T., Gronowitz, S. and Hoernfeldt, A.-B., On the syntheses of 8-heteroaryl-substituted 9-(?-D-ribofuranosyl)-2,6-diaminopurines through Pd-catalyzed coupling in the presence of cupric oxide, J. Heterocycl. Chem., 32 (1995) 863–866.
Brill, W.K.-D. and Riva-Toniolo, C., Solid phase synthesis of 2,6,8-trisubstituted purines, Tetrahedron Lett., 42 (2001) 6515–6518.
Moran, E.J., Sarshar, S., Cargill, J.F., Shahbaz, M.M., Lio, A., Mjalli, A.M.M. and Armstrong, R.W., Radio frequency tag encoded combinatorial library method for the discovery of tripeptide-substituted cinnamic acid inhibitors of the protein tyrosine phosphatase PTP1B, J. Am. Chem. Soc., 117 (1995) 10787–10788.
Furka, A., Sebestyen, F., Asgedom, M. and Dibo, G., General method for rapid synthesis of multicomponent peptide mixtures, Int. J. Pept. Protein Res., 37 (1991) 487–493.
Guiles, J.W., Lanter, C.L. and Rivero, R.A., A visual tagging process for mix and sort combinatorial chemistry, Angew. Chem. Int. Ed., 37 (1998) 926–928.
For instance: Zeng, L., Burton, L., Yung, K., Shushan, B. and Kassel, D.B., Automated analytical/preparative highperformance liquid chromatography-mass spectrometry system for the rapid characterization and purification of compound libraries, J. Chromatography A, 794 (1998) 3–13.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Riva-Toniolo, C., Müller, S., Schaub, J. et al. Catalysis of nucleophilic aromatic substitutions in the 2,6,8-trisubstituted purines and application in the synthesis of combinatorial libraries. Mol Divers 6, 43–53 (2003). https://doi.org/10.1023/A:1024895515316
Issue Date:
DOI: https://doi.org/10.1023/A:1024895515316