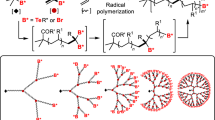
Abstract
Solid-phase dendrimer chemistry using a symmetrical 1→3C-branched isocyanate monomer was used to prepareradiation-grafted polymers with enhanced loading. Afterevaluation of the physical and chemical properties of thesenew high-loading supports, they were tested in the multipleparallel synthesis of hydantoins.
Similar content being viewed by others
References
Merrifield, R.B., Solid phase peptide synthesis. I. The synthesis of a tetrapeptide, J. Am. Chem. Soc., 85 (1963) 2149–2154.
Gallop, M.A., Barrett, R.W., Dower, W.J., Fodor, S.P.A. and Gordon, E.M., Applications of combinatorial technologies to drug discovery. 1._ Background and peptide combinatorial libraries, J. Med. Chem., 37 (1994) 1235–1251.
Bunin, B.A. and Ellman, J.A., A general and expedient method for the solid-phase synthesis of 1,4-benzodiazepine derivatives, J. Am. Chem. Soc., 114 (1992) 10997–10998.
DeWitt, S.H., Kiely, J.K., Stankovic, C.J., Schroeder, M.C., Cody, D.M.R. and Pavia, M.R., 'Diversomers': An approach to nonpeptide, nonoligomeric chemical diversity, Proc. Natl. Acad. Sci. U.S.A., 90 (1993) 6909–6913.
Geysen, H.M., Meloen, R.H. and Barteling, S.J., Use of peptide-synthesis to probe viral-antigens for epitopes to a resolution of a single amino-acid, Proc. Natl. Acad. Sci. Biol., 81 (1984) 3998–4002.
for leading reviews, see a) Pirrung, M.C., Spatially addressable combinatorial libraries, Chem. Rev., 97 (1997) 473–488.
Rinnová, M. and Lebl, M., Molecular diversity and libraries of structures: synthesis and screening, Collect. Czech. Chem. Commun., 61 (1996) 171–230.
Houghten, R.A., General method for the rapid solid-phase synthesis of large numbers of peptides: Specificity of antigenantibody interaction at the level of individual amino-acids, Proc. Natl. Acad. Sci. U.S.A., 82 (1985) 5131–5135.
Xiao, X.-Y., Li, R., Zhuang, H., Ewing, B., Karunaratne, K., Lillig, J., Brown, R. and Nicolaou, K.C., Solid-phase combinatorial synthesis using MicroKan reactors, Rf tagging, and directed sorting, Biotechnology and Bioengineering (Combinatorial Chemistry), 71 (2000) 44–50.
Lam, K.S., Lebl, M. and Krch?ák, V., The 'One-Bead-One-Compound' combinatorial library method, Chem. Rev., 97 (1997) 411–448.
Terrett, N.K., Gardner, M., Gordon, D.W., Kobylecki, R.J. and Steele, J., Combinatorial synthesis-The design of compound libraries and their application to drug discovery, Tetrahedron, 51 (1995) 8135–8173.
Atrash, B., Bradley, M., Kobylecki, R., Cowell, D. and Reader, J., Revolutionizing resin handling for combinatorial synthesis, Angew. Chem., Int. Ed. Engl., 40 (2001) 938–941.
Haag, R., Dendrimers and hyperbranched polymers as highloading supports for organic synthesis, Chem. Eur. J., 7 (2001) 327–335.
Literature references and technical notes on Synphase™ Crowns and Lanterns can be found on http:\\www.mimotopes.com\combichem\tech.html#articles.
Chow, H.-F., Mong, T.K.-K., Nongrum, M.F. and Wan, C.-W., The synthesis and properties of novel functional dendritic molecules, Tetrahedron, 54 (1998) 8543–8660.
Swali, V., Wells, N.J., Langley, G.J. and Bradley, M., Solid-phase dendrimer synthesis and the generation of superhigh-loading resin beads for combinatorial chemistry, J. Org. Chem., 62 (1997) 4902–4903.
Mahajan, A., Chhabra, S.R. and Chan,W.C., Resin-bound dendrimers as high loading supports for solid phase chemistry, Tetrahedron Lett., 40 (1999) 4909–4912.
Fromont, C. and Bradley, M., High-loading resin beads for solid phase synthesis using triple branching symmetrical dendrimers, Chem. Commun., (2000) 283–284.
Lebreton, S., Newcombe, N. and Bradley, M., Rapid synthesis of high-loading resins using triple branched protected monomer for dendrimer synthesis, Tetrahedron Lett., 43 (2002) 2475–2478.
Newkome, G.R., Weis, C.D. and Childs, B.J., Synthesis of 1?3 branched isocyanate monomers for dendritic construction, Designed Monomers and Polymers, 1 (1998) 3–14.
Sherrington, D.C., Preparation, structure and morphology of polymer supports, Chem. Commun., (1998) 2275–2286.
Sarin, V.K., Kent, S.B.H., Tam, J.P. and Merrifield, R.B., Quantitative monitoring of solid-phase peptide-synthesis by the ninhydrin reaction, Anal. Biochem., 117 (1981) 145–157.
Fields, G.B. and Noble, R.L., Solid phase peptide synthesis utilizing 9-fluorenylmethoxycarbonyl amino acids, Int. J. Peptide Protein Res., 35 (1990) 161–214.
Ihre, H., Padilla De JesÚs, O.L. and Fréchet, J.M.J., Fast and convenient divergent synthesis of aliphatic ester dendrimers by anhydride coupling, J. Am. Chem. Soc., 123 (2001) 5908–5917.
Based on the ninhydrin test, it was found that 72 hours were required to couple Fmoc-Gly-OH onto generation 1.0 (compared to 36 hours on resin beads) and 10 days for the second generation (compared to 5 days on resin beads).
Lebreton, S., Newcombe, N., Bradley, M., A novel 1?3 C-branched isocyanate monomer for resin amplification-a pseudo PS-PEG high-loading resin, Tetrahedron Lett., 43 (2002) 2479–2482.
Kim, S.W., Ahn, S.Y., Koh, J.S., Lee, J.H., Ro and S. Cho, H.Y., Solid phase synthesis of hydantoin library using a novel cyclization and traceless cleavage step, Tetrahedron Lett., 38 (1997) 4603–4606.
Kim, S.W., Koh, J.S., Lee, E.J. and Ro, S., Solid phase synthesis of benzamidine and butylamine-derived hydantoin libraries, Molecul. Div., 3 (1998) 129–132.
Hanessian, S. and Yang, R.Y., Solution and solid phase synthesis of 5-alkoxyhydantoin libraries with a three-fold functional diversity, Tetrahedron Lett., 37 (1996) 5835–5838.
Chong, P.Y. and Petillo, P., Solid phase hydantoin synthesis: An efficient and direct conversion of Fmoc-protected dipeptides to hydantoins, Tetrahedron Lett., 40 (1999) 2493–2496.
Dressman, B.A., Spangle, L.A. and Kaldor, S.W., Solid phase synthesis of hydantoins using a carbamate linker and a novel cyclization cleavage step, Tetrahedron Lett., 37 (1996) 937–940.
Lamothe, M., Lannuzel, M. and Perez, M., Solid-phase preparation of hydantoins though a new cyclization/cleavage step, J. Comb. Chem., 4 (2002) 73–78.
Park, K.-H., Ehrler, J., Spoerri, H. and Kurth, M., Preparation of a 990-member chemical compound library of hydantoinand isoxazoline-containing heterocycles using multipin technology, J. Comb. Chem., 3 (2001) 171–176.
Matthews, J. and Rivero, R.A., Base-promoted solid-phase synthesis of substituted hydantoins and thiohydantoins, J.Org. Chem., 62 (1997) 6090–6092.
Sheppard, R.C. and Williams, B.J., Acid-labile resin linkage agents for use in solid-phase peptide-synthesis, Int. J. Pept. Prot. Res., 20 (1982) 451–454.
Bui, C.T., Ercole, F., Pham, Y., Campbell, R., Rasoul, F.A., Maeji, N.J. and Ede, N.J., J. Peptide Sci. 2000, 6, 534–538.
MICROLUTE™ 96-well plate with bottom frit were purchased from Chromacol Ltd. This system is usually used for high-throughput Solid Phase Extraction (SPE).
Salvi, J.-P., Walchshofer, N. and Paris, J., Formation of bis(Fmoc-amino ethyl)-N-glycine derivatives by reductive amination of Fmoc-amino aldehydes with NaBH 3 CN, Tetrahedron Lett., 35 (1994) 1181–1184.
Yields were obtained by integral ratio using the phenolic protons and the two doublets of the methylene group coming from the benzaldehyde building block.
Hydantoin C: 1 H NMR (400 MHz, CDCl3) ? 0.84 (t, J= 7.5 Hz, 3H), ? 3.02 (dd, J= 4.5, 14.6 Hz, 1H), ? 3.09 (dd, J= 4.5, 14.6 Hz, 1H), ? 3.36 (m, 2H), ? 3.90 (t, J= 4.5 Hz, 1H), ? 3.91 (d, J= 15.1 Hz, 1H), ? 5.08 (d, J= 15.1 Hz, 1H), ? 7.03–7.07 (m, 4H), ? 7.18–7.27 (m, 6H); MS (ES+) m/e 363.2 (5%) (M+H)+, 747.4 (100%) (2M+Na)+. Hydantoin M: 1 H NMR (400 MHz, CDCl3) ? 1.42 (d, J= 7.0, 3H), ? 3.81 (s, 6H), ? 4.23 (q, J= 7.0, 1H), ? 4.45 (d, J= 15.6 Hz, 1H), ? 4.71 (d, J= 15.6 Hz, 1H), ? 6.50 (t, J= 2.0 Hz, 1H), ? 6.59 (d, J= 2.0 Hz, 2H), ? 7.45–7.58 (m, 5H), ? 6.97–7.06 (m, 6H); MS (ES+) m/e 341.2 (80%) (M+H)+, 703.3 (80%) (2M+Na)+.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lebreton, S., Newcombe, N. & Bradley, M. Loading amplification of radiation grafted polymers (crowns and lanterns) and their application in the solid-phase synthesis of hydantoin libraries. Mol Divers 6, 19–26 (2003). https://doi.org/10.1023/A:1024867013184
Issue Date:
DOI: https://doi.org/10.1023/A:1024867013184