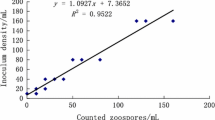
Abstract
Existing methods used to quantify microsclerotia of Verticillium dahliae in soil are reviewed. Most quantification methods are soil-type dependent, but are useful for disease prediction within certain soils. The major factor determining the accuracy of dry plating methods is the amount of soil plated per Petri dish. Wet plating methods are less sensitive to higher amounts of soil, especially when the fraction smaller than 20 um is removed by wet sieving. Despite general assumptions, wet plating methods do not have lower detection limits than dry plating methods. Dry plating methods are less variable at higher inoculum levels, but more variable at low inoculum levels. Bioassays are helpful tools in answering specific research questions, but are not convenient for large scale use. Molecular quantification techniques are promising, because they are not hampered by antagonistic effects, but data on their disease predictive abilities are still largely lacking. Suggestions are given for a better comparison of techniques, and some original results are presented to illustrate certain arguments.
Similar content being viewed by others
References
Andersen AA (1958) A new sampler for the collection, sizing and enumeration of viable air-borne particles. Journal of Bacteriology 76: 471
Ashworth LJ, Harper DM and Andris HL (1974) The influence of milling air-dry soil upon apparent inoculum density and propagule size of Verticillium albo-atrum. Phytopathology 64: 629-632
Ashworth LJ, Huisman OC, Grogan RG and Harper DM (1976) Copper induced fungistasis of microsclerotia of Verticillium albo-atrum and its influence on infection of cotton in the field. Phytopathology 66: 970-977
Ashworth LJ, McCutcheon OD and George AG (1972a) Verticillium albo-atrum: The quantitative relationship between inoculum density and infection of cotton. Phytopathology 62: 901-903
Ashworth LJ, Waters JE, George AG and McCutcheon OD (1972b) Assessment of microsclerotia of Verticillium albo-atrum in field soils. Phytopathology 62: 715-719
Aspromougos I and Schlösser E (2000) A competitive PCR assay to quantify Verticillium dahliae in infected, resistant and susceptible tomato hybrids with HPLC. In: Tjamos EC, Rowe RC, Heale JB and Fravel DR (eds) Advances in Verticillium: Research and Disease Management (pp 44-47) APS Press, St. Paul
Ben-Yephet Y and Pinkas Y (1976) A cesium chloride flotation technique for the isolation of Verticillium dahliae microsclerotia from soil. Phytopathology 66: 1252-1254
Ben-Yephet Y and Pinkas Y (1977) Germination of individual microsclerotia of Verticillium dahliae. Phytoparasitica 5: 159-166
Berg G and Ballin G (1994) Bacterial antagonists to Verticillium dahliae Kleb. Journal of Phytopathology 141: 99-110
Bollen GJ, Hoekstra O, Scholte K, Hofman TW, Celetti MJ and Schirring A (1989) Incidence of soilborne pathogens in potato related to the frequency of potato growing on a clay loam. In: Vos J, van Loon CD and Bollen GJ (eds) Effects of Crop Rotation on Potato Production in the Temperate Zones (pp 203-222) Kluwer Academic Publishers, Dordrecht
Butterfield EJ and DeVay JE (1977) Reassessment of soil assays for Verticillium dahliae. Phytopathology 67: 1073-1078
Buxton EW and Kendrick JB (1963) A method of isolating Pythium and Fusarium from soil. Annals of Applied Biology 51: 215-221
Camporota P and Rouxel F (1977) Intérêt du broyage et du tamisage à sec dans les techniques d'isolement du Verticillium dahliae à partir du sol. Annales de Phytopathologie 9: 77-81
Carder JH, Morton AA, Tabrett AM and Barbara DJ (1994) Detection and differentiation by PCR of subspecific groups within two Verticillium species causing vascular wilts in herbaceous hosts. In: Schots A, Dewey FM and Oliver R (eds) Modern Assays for Plant Pathogenic Fungi: Identification, Detection and Quantification (pp 91-97) CAB International, Wallingford
Davis JR, Pavek JJ and Corsini DL (1983) A sensitive method for quantifyingVerticillium dahliae colonization in plant tissue and evaluating resistance among potato genotypes. Phytopathology 73: 1009-1014
DeVay JE, Forrester LL, Garber RH and Butterfield EJ (1974) Characteristics and concentration of propagules of Verticillium dahliae in air-dried field soils in relation to the prevalence of verticillium wilt in cotton. Phytopathology 64: 22-29
Emmatty DA and Green RJ (1969) Fungistasis and the behavior of the microsclerotia of Verticillium albo-atrum in soil. Phytopathology 59: 1590-1595
Evans G and Gleeson AC (1973) Observations on the origin and nature of Verticillium dahliae colonizing plant roots. Australian Journal of Biological Sciences 26: 151-161
Evans G, McKeen CD and Gleeson AC (1974) A quantitative bioassay for determining low numbers of microsclerotia of Verticillium dahliae in field soils. Canadian Journal of Microbiology 20: 119-124
Evans G, Wilhelm S and Snyder WC (1967) Quantitative studies by plate counts of propagules of the Verticillium wilt fungus in cotton field soils. Phytopathology 57: 1250-1255
Goud JC, Termorshuizen AJ, Blok WJ and Coenen GCM (2000) Biologische grondontsmetting en de invloed van initiële inoculumdichtheid op de ontwikkeling van Verticillium dahliae in Noorse esdoorn en trompetboom. Gewasbescherming 31: 13
Green RJ and Papavizas GC (1968) The effect of carbon source, carbon to nitrogen ratios, and organic amendments on survival of propagules of Verticillium albo-atrum in soil. Phytopathology 58: 567-570
Harris DC (1998) An introduction to verticillium wilts. In: Hiemstra JA and Harris DC (eds) A Compendium of Verticillium Wilts in Tree Species (pp 1-4) CPRO-DLO/HRI-EM, Wageningen/West Malling
Harris DC and Yang YR (1996) The relationship between the amount of Verticillium dahliae in soil and the incidence of strawberry wilt as a basis for disease risk prediction. Plant Pathology 45: 106-114
Harris DC, Yang YR and Ridout MS (1993) The detection and estimation of Verticillium dahliae in naturally infested soil. Plant Pathology 42: 238-250
Harrison HD and Livingston CH (1966) A method for isolating Verticillium from field soil. Plant Disease Reporter 50: 897-899
Haverkort AJ, Vos J, Groenwold J and Hoekstra G (1989) Crop characteristics and yield reduction of potato due to biotic stress in short crop rotations. In: Vos J, van Loon CD and Bollen GJ (eds) Effects of Crop Rotation on Potato Production in the Temperate Zones (pp 273-290) Kluwer Academic Publishers, Dordrecht
Hawke MA and Lazarovits G (1994) Production and manipulation of individual microsclerotia of Verticillium dahliae for use in studies of survival. Phytopathology 84: 883-890
Heinz RA and Platt HW (2000) A competitive PCR-based assay to quantify Verticillium tricorpus propagules in soil. Canadian Journal of Plant Pathology 22: 122-130
Heppner C and Heitefuss R (1997) Development of an enzymelinked immunosorbent assay (ELISA) for Verticillium dahliae detection in soil. In: Dehne HW, Adam G, Diekmann M, Frahm J, Mauler-Machnik A and van Halteren P (eds) Diagnosis and Identification of Plant Pathogens. Proceedings of the 4th International Symposium of the European Foundation for Plant Pathology, Bonn, Germany, 9-12 September 1996 (pp 105-108) Kluwer Academic Publishers, Dordrecht
Hoyos GP, Zambino PJ and Anderson NA (1991) An assay to quantify vascular colonization of potato by Verticillium dahliae. American Potato Journal 68: 727-742
Hu X, Nazar RN and Robb J (1993) Quantification of Verticillium biomass in wilt disease development. Physiological and Molecular Plant Pathology 42: 23-36
Huisman OC (1988) Seasonal colonization of roots of field-grown cotton by Verticillium dahliae and V. tricorpus. Phytopathology 78: 708-716
Huisman OC and Ashworth LJ (1974a) Quantitative assessment of Verticillium albo-atrum in field soils: Procedural and substrate improvements. Phytopathology 64: 1043-1044
Huisman OC and Ashworth LJ (1974b) Verticillium albo-atrum: Quantitative isolation of microsclerotia from field soils. Phytopathology 64: 1159-1163
Isaac I (1967) Speciation in Verticillium. Annual Review of Phytopathology 5: 201-222
Isaac I, Fletcher P and Harrison JAC (1971) Quantitative isolation of Verticillium spp. from soil and moribund potato haulm. Annals of Applied Biology 67: 177-183
Jiménez-Díaz RM, Tjamos EC and Cirulli M (1998) Verticillium wilts of major tree hosts. Olive. In: Hiemstra JA and Harris DC (eds) A Compendium of Verticillium Wilts in Tree Species (pp 13-16) CPRO-DLO/HRI-EM, Wageningen/West Malling
Kabir Z, Bhat RG and Subbarao KV (2001) Optimization of polygalacturonic acid with NaOH in NP-10 medium to improve Verticillium dahliae recovery from soil. Book of Abstracts, 8th International Verticillium Symposium, Cordoba, Spain, 5-8 November 2001, p 71
Kapulnik E, Quick J and DeVay JE (1975) Germination of propagules of Verticillium dahliae in soil treated with methionine and other substances affecting ethylene production. Phytopathology 75: 1348
Komada H (1975) Development of a selective medium for quantitative isolation of Fusarium oxysporum from natural soil. Review of Plant Protection Research 8: 114-124
Krishnamurthy V, Barbara D and Price A (2001) PCR-based quantification of Verticillium dahliae in soil. Book of Abstracts, 8th International Verticillium Symposium, Cordoba, Spain, 5-8 November 2001, p 73
Li KN, Rouse DI, Eyestone EJ and German TL (1999) The generation of specific DNA primers using random amplified polymorphic DNA and its application to Verticillium dahliae. Mycological Research 103: 1361-1368
Li KN, Rouse DI and German TL (1994) PCR primers that allow intergeneric differentiation of Ascomycetes and their application to Verticillium spp. Applied and Environmental Microbiology 60: 4324-4331
Locke T (2001) The effect of V. dahliae on potato production in England and Wales. Book of Abstracts, 8th International Verticillium Symposium, Cordoba, Spain, 5-8November 2001, p 77
Mahuku GS and Platt HW (2002) Quantifying Verticillium dahliae in soils collected from potato fields using a competitive PCR assay. American Journal of Potato Research 79: 107-117
Mahuku GS, Platt HW and Maxwell P (1999) Comparison of polymerase chain reaction methods with plating on media to detect and identify Verticillium wilt pathogens on potato. Canadian Journal of Plant Pathology 21: 125-131
Maloy OC and Alexander M (1958) The 'most probable number' method for estimating populations of plant pathogenic organisms in the soil. Phytopathology 48: 126-128
Marois JJ, Johnston SA, Dunn MT and Papavizas GC (1982) Biological control of verticillium wilt of eggplant in the field. Plant Disease 66: 1166-1168
Martin MJ, Riedel RM and Rowe RC (1982) Verticillium dahliae and Pratylenchus penetrans: Interactions in the early dying complex of potatoes in Ohio. Phytopathology 72: 640-644
Menzies JD and Griebel GE (1967) Survival and saprophytic growth of Verticillium dahliae in uncropped soil. Phytopathology 57: 703-709
Mercado-Blanco J, Rodríguez-Jurado D, Pérez-Artés E and Jimenez-Díaz RM (2001) Detection of the nondefoliating pathotype of Verticillium dahliae in infected olive plants by nested PCR. Plant Pathology 50: 609-619
Nadakavukaren MJ and Horner CE (1959) An alcohol agar medium selective for determining Verticillium microsclerotia in soil. Phytopathology 49: 527-528
Nagtzaam MPM (1998) Biological Control of Verticillium dahliae by Talaromyces flavus. PhD Thesis, Wageningen University, Wageningen
Nagtzaam MPM, Termorshuizen AJ and Bollen GJ (1997) The relationship between soil inoculum density and plant infection as a basis for a quantitative bioassay of Verticillium dahliae. European Journal of Plant Pathology 103: 597-605
Nazar RN, Hu X, Schmidt D, Culham D and Robb J (1991) Potential use of PCR-amplified ribosomal intergenic sequences in the detection and differentiation of verticillium wilt pathogens. Physiological and Molecular Plant Pathology 39: 1-11
Nicot PC and Rouse DI (1987a) Precision and bias of three quantitative soil assays for Verticillium dahliae. Phytopathology 77: 875-881
Nicot PC and Rouse DI (1987b) Relationship between soil inoculum density of Verticillium dahliae and systemic colonization of potato stems in commercial fields over time. Phytopathology 77: 1346-1355
Olsson S and Nordbring-Hertz B (1985) Microsclerotial germination of Verticillium dahliae as affected by rape rhizosphere. FEMS Microbiology Ecology 31: 293-299
Palloix A, Pochard E, Phaly T and Daubèze AM (1990) Recurrent selection for resistance to Verticillium dahliae in pepper. Euphytica 47: 79-89
Paplomatas EJ, Bassett DM, Broome JC and DeVay JE (1992) Incidence of Verticillium wilt and yield losses of cotton cultivars (Gossypium hirsutum) based on soil inoculum density of Verticillium dahliae. Phytopathology 82: 1417-1420
Pegg GF and Brady BL (2002) Verticillium Wilts. CABI Publishing, Wallingford
Pérez-Artés E, Ruz Carrillo A and Jiménez-Díaz RM (2001) PCR-based detection of defoliating and nondefoliating pathotypes of Verticillium dahliae in soil. Book of Abstracts, 8th International Verticillium Symposium, Cordoba, Spain, 5-8 November 2001, p 60
Platt HW, Mahuku GS, Maxwell P and MacLean V (2000) Detection techniques for research on Verticillium species in potato soils. In: Tjamos EC, Rowe RC, Heale JB and Fravel DR (eds) Advances in Verticillium: Research and Disease Management (pp 140-143) APS Press, St. Paul
Powelson RL (1966) Availability of diffusable nutrients for germination and growth of Verticillium dahliae in soils amended with oat and alfalfa residues. Phytopathology 56: 895
Robb J and Nazar RN (1997) Application of PCR-based diagnostics to monitor Verticillium-nematode disease complexes in the field. Book of Abstracts, 7th International Verticillium Symposium, Cape Sounion, Athens, Greece, 6-10 October 1997, p 16
Robb J, Moukhamedov R, Hu X, Platt HW and Nazar RN (1993) Putative subgroups of Verticillium albo-atrum distinguishable by PCR-based assays. Physiological and Molecular Plant Pathology 43: 423-436
Robb J, Hu X, Platt HW and Nazar RN (1994) PCR-based assays for the detection and quantification of Verticillium species in potato. In: Schots A, Dewey FM and Oliver R (eds) Modern Assays for Plant Pathogenic Fungi: Identification, Detection and Quantification (pp 83-90) CAB International, Wallingford
Rush CM, Mihail JD and Singleton LL (1992) Introduction. In: Singleton LL, Mihail JD and Rush CM (eds) Methods for Research on Soilborne Phytopathogenic Fungi (pp 3-6) APS Press, St. Paul
Schnathorst WC and Fogle D (1973) Survival structures of Verticillium dahliae in naturally infested field soils. Proceedings of the Beltwide Cotton Production Research Conference, Phoenix, Arizona, 9-10 January, 1973, p 24
Schreiber LR and Green RJ (1963) Effect of root exudates on germination of conidia and microsclerotia of Verticillium albo-atrum inhibited by the soil fungistatic principle. Phytopathology 53: 260-264
Smith VL and Rowe RC (1984) Characteristics and distribution of propagules of Verticillium dahliae in Ohio potato field soils and assessment of two assay methods. Phytopathology 74: 553-556
Soesanto L (2000) Ecology and Biological Control of Verticillium dahliae. PhD Thesis, Wageningen University, Wageningen
Sorensen LH, Schneider AT and Davis JR (1991) Influence of sodium polygalacturonate sources and improved recovery of Verticillium spp. from soil. Phytopathology 81: 1347
Stanghellini ME, von Bretzel P, Kronland WC and Jenkins AD (1982) Inoculum densities of Pythium aphanidermatum in soils of irrigated sugar beet fields in Arizona. Phytopathology 72: 1481-1485
Taylor JB (1969) Fluctuations in natural soil populations of Verticillium tricorpus. Canadian Journal of Botany 47: 737-740
Termorshuizen AJ, Davis JR, Gort G, Harris DC, Huisman OC, Lazarovits G, Locke T, MeleroVara JM, Mol L, Paplomatas EJ, Platt HW, Powelson M, Rouse DI, Rowe RC and Tsror L (1998) Interlaboratory comparison of methods to quantify microsclerotia of Verticillium dahliae in soil. Applied and Environmental Microbiology 64: 3846-3853
Termorshuizen AJ, Davis JR, Gort G, Harris DC, Huisman OC, Lazarovits G, Locke T, Melero Vara JM, Mol L, Paplomatas EJ, Platt HW, Powelson M, Rouse DI, Rowe RC and Tsror L (1999) Interlaboratory comparison of methods to quantify microsclerotia of Verticillium dahliae in soil. Applied and Environmental Microbiology 65: 2805 (Erratum)
Tsai SD and Erwin DC (1975) A method of quantifying numbers of microsclerotia of Verticillium albo-atrum in cotton plant tissue and in pure culture. Phytopathology 65: 1027-1028
Wheeler TA, Madden LV, Riedel RM and Rowe RC (1994) Distribution and yield-loss relations of Verticillium dahliae, Pratylenchus penetrans, P. scribneri, P. crenatus, and Meloidogyne hapla in commercial potato fields. Phytopathology 84: 843-852
Zehsazian H (1966) An Improved Selective Medium for the Isolation of Verticillium dahliae Kleb. MSc Thesis, Oregon State University, Corvallis
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Goud, J., Termorshuizen, A. Quality of Methods to Quantify Microsclerotia of Verticillium dahliae in Soil. European Journal of Plant Pathology 109, 523–534 (2003). https://doi.org/10.1023/A:1024745006876
Issue Date:
DOI: https://doi.org/10.1023/A:1024745006876