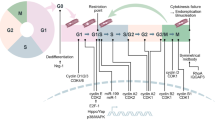
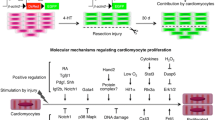
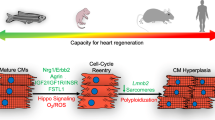
Abstract
Lower vertebrates such as newt and zebrafish are able to reactivate high levels of cardiomyocyte cell cycle activity in response to experimental injury resulting in apparent regeneration. In contrast, damaged myocardium is replaced by fibrotic scar tissue in higher vertebrates. This process compromises the contractile function of the surviving myocardium, ultimately leading to heart failure. Various strategies are being pursued to augment myocyte number in the diseased hearts. One approach entails the reactivation of cell cycle in surviving cardiomyocytes. Here, we provide a summary of methods to monitor cell cycle activity, and interventions demonstrating positive cell cycle effects in cardiomyocytes as well as discuss the potential utility of cell cycle regulation to augment myocyte number in diseased hearts.
Similar content being viewed by others
References
Planas-Silva MD, Weinberg RA. The restriction point and control of cell proliferation. Curr Opin Cell Biol 1997;9(6):768-772.
Hunter T, Pines J. Cyclins, cancer. II: Cyclin D and CDK inhibitors come of age. Cell 1994;79(4):573-582.
Malumbres M, Barbacid M. To cycle or not to cycle: A critical decision in cancer. Nat Rev Cancer 2001;1(3):222-231.
King RW, Jackson PK, Kirschner MW. Mitosis in transition. Cell 1994;79(4):563-571.
Heichman KA, Roberts JM. Rules to replicate by. Cell 1994;79(4):557-562.
Sherr CJ. G1 phase progression: Cycling on cue. Cell 1994;79(4):551-555.
Nurse P. Ordering S phase and M phase in the cell cycle. Cell 1994;79(4):547-550.
Pines J, Rieder CL. Re-staging mitosis: A contemporary view of mitotic progression. Nat Cell Biol 2001;3(1):E3- E6.
Koepp DM, Harper JW, Elledge SJ. How the cyclin became a cyclin: Regulated proteolysis in the cell cycle. Cell 1999;97(4):431-434.
Oberpriller JO, Oberpriller JC. Response of the adult newt ventricle to injury. J Exp Zool 1974;187(2):249-253.
Oberpriller JO, Oberpriller JC, Arefyeva AM, et al. Nuclear characteristics of cardiac myocytes following the proliferative response to mincing of the myocardium in the adult newt, Notophthalmus viridescens. Cell Tissue Res 1988;253(3):619-624.
Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science 2002;298(5601):2188-2190.
Rumyantsev PP. Growth and Hyperplasia of Cardiac Muscle Cells. New York City, NY: Harwood Academic Publishers, 1991.
Soonpaa MH, Kim KK, Pajak L, et al. Cardiomyocyte DNA synthesis and binucleation during murine development. Am J Physiol 1996;271(5 Pt 2):H2183-2189.
Clubb FJ, Jr., Bishop SP. Formation of binucleated myocardial cells in the neonatal rat. An index for growth hypertrophy. Lab Invest 1984;50(5):571-577.
Li F, Wang X, Capasso JM, Gerdes AM. Rapid transition of cardiac myocytes from hyperplasia to hypertrophy during postnatal development. J Mol Cell Cardiol 1996;28(8):1737-1746.
Pasumarthi KB, Field LJ. Cardiomyocyte cell cycle regulation. Circ Res 2002;90(10):1044-1054.
Soonpaa MH, Field LJ. Survey of studies examining mammalian cardiomyocyte DNA synthesis. Circ Res 1998;83(1):15-26.
Soonpaa MH, Koh GY, Klug MG, Field LJ. Formation of nascent intercalated disks between grafted fetal cardiomyocytes and host myocardium. Science 1994;264(5155):98-101.
Eldridge SR, Goldsworthy SM. Cell proliferation rates in common cancer target tissues of B6C3F1 mice and F344 rats: Effects of age, gender, and choice of marker. Fundam Appl Toxicol 1996;32(2):159-167.
Maiolino P, Restucci B, De Vico G. Expression of proliferating cell nuclear antigen in basal cell carcinomas and in squamous cell carcinomas of canine skin: Correlation with mitotic index and histological features. Zentralbl Veterinarmed A 1995;42(5):339-343.
Connolly KM, Bogdanffy MS. Evaluation of proliferating cell nuclear antigen (PCNA) as an endogenous marker of cell proliferation in rat liver: A dual-stain comparison with 5-bromo-2'-deoxyuridine. J Histochem Cytochem 1993;41(1):1-6.
Chapman DL, Wolgemuth DJ. Expression of proliferating cell nuclear antigen in the mouse germ line and surrounding somatic cells suggests both proliferationdependent and-independent modes of function. Int J Dev Biol 1994;38(3):491-497.
Pandey S, Wang E. Cells en route to apoptosis are characterized by the upregulation of c-fos, c-myc, c-jun, cdc2, and RB phosphorylation, resembling events of early cell-cycle traverse. J Cell Biochem 1995;58(2):135-150.
Hall PA, Coates PJ, Goodlad RA, et al. Proliferating cell nuclear antigen expression in non-cycling cells may be induced by growth factors in vivo. Br J Cancer 1994;70(2):244-247.
Scholzen T, Gerdes J. The Ki-67 protein: From the known and the unknown. J Cell Physiol 2000;182(3):311-322.
MacCallum DE, Hall PA. The location of pKi67 in the outer dense fibrillary compartment of the nucleolus points to a role in ribosome biogenesis during the cell division cycle. J Pathol 2000;190(5):537-544.
van Oijen MG, Medema RH, Slootweg PJ, Rijksen G. Positivity of the proliferation marker Ki-67 in noncycling cells. Am J Clin Pathol 1998;110(1):24-31.
Warnakulasuriya KA, Johnson NW. Comparison of isotopic and immunohistochemical methods of studying epithelial cell proliferation in hamster tongue. Cell Prolif 1993;26(6):545-555.
van Weerden WM, Moerings EP, van Kreuningen A, et al. Ki-67 expression and BrdUrd incorporation as markers of proliferative activity in human prostate tumour models. Cell Prolif 1993;26(1):67-75.
Boon ME, Howard CV, van Velzen D. PCNA independence of Ki-67 expression in HPV infection. Cell Biol Int 1993;17(11):1001-1004.
Uma Devi P, Satish Rao BS, Kamath R. A method to score micronuclei in vivo using cytochalasin B-induced cytokinesis block. Mutat Res 1998;401(1/2):33-37.
Bousquet PF, Paulsen LA, Fondy C, et al. Effects of cytochalasin B in culture and in vivo on murine Madison 109 lung carcinoma and on B16 melanoma. Cancer Res 1990;50(5):1431-1439.
Soonpaa MH, Field LJ. Assessment of cardiomyocyte DNAsynthesis in normal and injured adult mouse hearts. Am J Physiol 1997;272(1 Pt 2):H220-226.
Zhao YY, Sawyer DR, Baliga RR, et al. Neuregulins promote survival and growth of cardiac myocytes. Persistence of ErbB2 and ErbB4 expression in neonatal and adult ventricular myocytes. J Biol Chem 1998;273(17):10261-10269.
Cohen-Gould L, Mikawa T. The fate diversity of mesodermal cells within the heart field during chicken early embryogenesis. Dev Biol 1996;177(1):265-273.
Engelmann GL, Birchenall-Roberts MC, Ruscetti FW, Samarel AM. Formation of fetal rat cardiac cell clones by retroviral transformation: Retention of select myocyte characteristics. J Mol Cell Cardiol 1993;25(2):197-213.
Sen A, Dunnmon P, Henderson SA, et al. Terminally differentiated neonatal rat myocardial cells proliferate and maintain specific differentiated functions following expression of SV40 large T antigen. J Biol Chem 1988;263(35):19132-19136.
Liu Y, Kitsis RN. Induction of DNA synthesis and apoptosis in cardiac myocytes by E1A oncoprotein. J Cell Biol 1996;133(2):325-334.
Kirshenbaum LA, Schneider MD. Adenovirus E1A represses cardiac gene transcription and reactivates DNA synthesis in ventricular myocytes, via alternative pocket protein-and p300-binding domains. J Biol Chem 1995;270(14):7791-7794.
Agah R, Kirshenbaum LA, Abdellatif M, et al. Adenoviral delivery of E2F-1 directs cell cycle reentry and p53-independent apoptosis in postmitotic adult myocardium in vivo. J Clin Invest 1997;100(11):2722-2728.
Su H, Lu R, Ding R, Kan YW. Adeno-associated viralmediated gene transfer to hepatoma: Thymidine kinase/ interleukin 2 is more effective in tumor killing in non-ganciclovir (GCV)-treated than in GCV-treated animals. Mol Ther 2000;1(6):509-515.
Maeda Y, Ikeda U, Shimpo M, et al. Efficient gene transfer into cardiac myocytes using adeno-associated virus (AAV) vectors. J Mol Cell Cardiol 1998;30(7):1341-1348.
Sakoda T, Kasahara N, Hamamori Y, Kedes L. Ahigh-titer lentiviral production system mediates efficient transduction of differentiated cells including beating cardiac myocytes. J Mol Cell Cardiol 1999;31(11):2037-2047.
Mitta B, Rimann M, Ehrengruber MU, et al. Advanced modular self-inactivating lentiviral expression vectors for multigene interventions in mammalian cells and in vivo transduction. Nucleic Acids Res 2002;30(21):e113.
Snyder RO, Flotte TR. Production of clinical-grade recombinant adeno-associated virus vectors. Curr Opin Biotechnol 2002;13(5):418-423.
Hermens WT, Verhaagen J. Viral vectors, tools for gene transfer in the nervous system. Prog Neurobiol 1998;55(4):399-432.
Buttrick PM, Kass A, Kitsis RN, et al. Behavior of genes directly injected into the rat heart in vivo. Circ Res 1992;70(1):193-198.
Lin H, Parmacek MS, Morle G, et al. Expression of recombinant genes in myocardium in vivo after direct injection of DNA. Circulation 1990;82(6):2217-2221.
Poston RS, Tran KP, Mann MJ, et al. Prevention of ischemically induced neointimal hyperplasia using ex vivo antisense oligodeoxynucleotides. J Heart Lung Transplant 1998;17(4):349-355.
Zhu N, Liggitt D, Liu Y, Debs R. Systemic gene expression after intravenous DNA delivery into adult mice. Science 1993;261(5118):209-211.
Shi Y, Fard A, Galeo A, et al. Transcatheter delivery of c-myc antisense oligomers reduces neointimal formation in a porcine model of coronary artery balloon injury. Circulation 1994;90(2):944-951.
Simons M, Edelman ER, DeKeyser JL, et al. Antisense c-myb oligonucleotides inhibit intimal arterial smooth muscle cell accumulation in vivo. Nature 1992;359(6390): 67-70.
Morishita R, Yamada S, Yamamoto K, et al. Novel therapeutic strategy for atherosclerosis: Ribozyme oligonucleotides against apolipoprotein(a) selectively inhibit apolipoprotein(a) but not plasminogen gene expression. Circulation 1998;98(18):1898-1904.
Yamamoto K, Morishita R, Tomita N, et al. Ribozyme oligonucleotides against transforming growth factorbeta inhibited neointimal formation after vascular injury in rat model: Potential application of ribozyme strategy to treat cardiovascular disease. Circulation 2000;102(11):1308-1314.
Sawa Y, Morishita R, Suzuki K, et al. A novel strategy for myocardial protection using in vivo transfection of cis element 'decoy' against NFkappaB binding site: Evidence for a role of NFkappaB in ischemia-reperfusion injury. Circulation 1997;96(9 Suppl):II-280-284; discussion II-285.
Morishita R, Gibbons GH, Horiuchi M, et al. A gene therapy strategy using a transcription factor decoy of the E2F binding site inhibits smooth muscle proliferation in vivo. Proc Natl Acad Sci USA 1995;92(13):5855-5859.
Morishita R, Sugimoto T, Aoki M, et al. In vivo transfection of cis element "decoy" against nuclear factor-kappaB binding site prevents myocardial infarction. Nat Med 1997;3(8):894-899.
Ito WD, Arras M, Winkler B, et al. Monocyte chemotactic protein-1 increases collateral and peripheral conductance after femoral artery occlusion. Circ Res 1997;80(6):829-837.
Schwarze SR, Hruska KA, Dowdy SF. Protein transduction: Unrestricted delivery into all cells? Trends Cell Biol 2000;10(7):290-295.
Dowell JD, Rubart M, Pasumarthi KB, et al. Myocyte and myogenic stem cell transplantation in the heart. Cardiovascular Research 2003;58(2):336-350.
Huh NE, Pasumarthi KB, Soonpaa MH, et al. Functional abrogation of p53 is required for T-Ag induced proliferation in cardiomyocytes. J Mol Cell Cardiol 2001;33(8):1405-1419.
Pasumarthi KB, Tsai SC, Field LJ. Coexpression of mutant p53 and p193 renders embryonic stem cell-derived cardiomyocytes responsive to the growth-promoting activities of adenoviral E1A. Circ Res 2001;88(10):1004-1011.
Kawakami Y, Yamaoka T, Hirochika R, et al. Somatic gene therapy for diabetes with an immunological safety 302 Dowell et al. system for complete removal of transplanted cells. Diabetes 1992;41(8):956-961.
Hoogerbrugge PM, Vossen JM, v Beusechem VW, Valerio D. Treatment of patients with severe combined immunodeficiency due to adenosine deaminase (ADA) deficiency by autologous transplantation of genetically modified bone marrow cells. Hum Gene Ther 1992;3(5): 553-558.
Koh GY, Kim SJ, Klug MG, et al. Targeted expression of transforming growth factor-beta 1 in intracardiac grafts promotes vascular endothelial cell DNA synthesis. J Clin Invest 1995;95(1):114-121.
Komarov PG, Komarova EA, Kondratov RV, et al. A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science 1999;285(5434):1733-1737.
Rapisarda A, Uranchimeg B, Scudiero DA, et al. Identification of small molecule inhibitors of hypoxia-inducible factor 1 transcriptional activation pathway. Cancer Res 2002;62(15):4316-4324.
Zaharevitz DW, Gussio R, Leost M, et al. Discovery and initial characterization of the paullones, a novel class of small-molecule inhibitors of cyclin-dependent kinases. Cancer Res 1999;59(11):2566-2569.
Lightstone FC, Prieto MC, Singh AK, et al. Identification of novel small molecule ligands that bind to tetanus toxin. Chem Res Toxicol 2000;13(5):356-362.
Massa SM, Xie Y, Longo FM. Alzheimer's therapeutics: Neurotrophin small molecule mimetics. J Mol Neurosci 2002;19(1/2):107-111.
Soonpaa MH, Oberpriller JO, Oberpriller JC. Factors altering DNA synthesis in the cardiac myocyte of the adult newt, Notophthalmus viridescens. Cell Tissue Res 1994;275(2):377-382.
Vara DJ, Bicknell KA, Head K. E2F3 plays a key role in cardiac myocyte growth. Circulation 2002;106(19):II-141.
Kardami E. Stimulation and inhibition of cardiac myocyte proliferation in vitro. Mol Cell Biochem 1990;92(2):129-135.
Steinhelper ME, Lanson NA, Jr., Dresdner KP, et al. Proliferation in vivo and in culture of differentiated adult atrial cardiomyocytes from transgenic mice. Am J Physiol 1990;259(6 Pt 2):H1826-1834.
Field LJ. Atrial natriuretic factor-SV40 T antigen transgenes produce tumors and cardiac arrhythmias in mice. Science 1988;239(4843):1029-1033.
Reiss K, Cheng W, Pierzchalski P, et al. Insulin-like growth factor-1 receptor and its ligand regulate the reentry of adult ventricular myocytes into the cell cycle. Exp Cell Res 1997;235(1):198-209.
Gartside CL, Hauschka SD. The Development and Regenerative Potential of Cardiac Muscle. London, UK: Harwood, 1991.
Behringer RR, Peschon JJ, Messing A, et al. Heart and bone tumors in transgenic mice. Proc Natl Acad Sci USA 1988;85(8):2648-2652.
Goldman B, Mach A, Wurzel J. Epidermal growth factor promotes a cardiomyoblastic phenotype in human fetal cardiac myocytes. Exp Cell Res 1996;228(2):237-245.
Katz EB, Steinhelper ME, Delcarpio JB, et al. Cardiomyocyte proliferation in mice expressing alpha-cardiac myosin heavy chain-SV40 T-antigen transgenes. Am J Physiol 1992;262(6 Pt 2):H1867-1876.
Sheng Z, Pennica D, Wood WI, Chien KR. Cardiotrophin-1 displays early expression in the murine heart tube and promotes cardiac myocyte survival. Development 1996;122(2):419-428.
Brunskill EW, Witte DP, Yutzey KE, Potter SS. Novel cell lines promote the discovery of genes involved in early heart development. Dev Biol 2001;235(2):507-520.
Rybkin II, Markham DW, Yan Z, et al. Conditional expression of SV40 T antigen in mouse cardiomyocytes facilitates an inducible switch from proliferation to differentiation. J Biol Chem 2003.
De Leon JR, Federoff HJ, Dickson DW, et al. Cardiac and skeletal myopathy in beta myosin heavy-chain simian virus 40 tsA58 transgenic mice. Proc Natl Acad Sci USA 1994;91(2):519-523.
Palmer JN, Hartogensis WE, Patten M, et al. Interleukin-1 beta induces cardiac myocyte growth but inhibits cardiac fibroblast proliferation in culture. J Clin Invest 1995;95(6):2555-2564.
Koide M, Akins RE, Harayama H, et al. Atrial natriuretic peptide accelerates proliferation of chick embryonic cardiomyocytes in vitro. Differentiation 1996;61(1):1-11.
Jackson T, Allard MF, Sreenan CM, et al. The c-myc proto-oncogene regulates cardiac development in transgenic mice. Mol Cell Biol 1990;10(7):3709-3716.
Machida N, Brissie N, Sreenan C, Bishop SP. Inhibition of cardiac myocyte division in c-myc transgenic mice. J Mol Cell Cardiol 1997;29(7):1895-1902.
Lau CL. Behavior of embryonic chick heart cells in culture. 1. Cellular responses to insulin-transferrinselenium. Tissue Cell 1993;25(4):465-480.
Xiao G, Mao S, Baumgarten G, et al. Inducible activation of c-Myc in adult myocardium in vivo provokes cardiac myocyte hypertrophy and reactivation of DNA synthesis. Circ Res 2001;89(12):1122-1129.
Claycomb WC, Moses RL. Growth factors and TPA stimulate DNA synthesis and alter the morphology of cultured terminally differentiated adult rat cardiac muscle cells. Dev Biol 1988;127(2):257-265.
Soonpaa MH, Koh GY, Pajak L, et al. Cyclin D1 overexpression promotes cardiomyocyte DNA synthesis and multinucleation in transgenic mice. J Clin Invest 1997;99(11):2644-2654.
Wald MR, Borda ES, Sterin-Borda L. Mitogenic effect of erythropoietin on neonatal rat cardiomyocytes: Signal transduction pathways. J Cell Physiol 1996;167(3):461-468.
Liao HS, Kang PM, Nagashima H, et al. Cardiacspecific overexpression of cyclin-dependent kinase 2 increases smaller mononuclear cardiomyocytes. Circ Res 2001;88(4):443-450.
Pasumarthi KB, Doble BW, Kardami E, Cattini PA. Overexpression of CUG-orAUG-initiated forms of basic fibroblast growth factor in cardiac myocytes results in similar effects on mitosis and protein synthesis but distinct nuclear morphologies. J Mol Cell Cardiol 1994;26(8):1045-1060.
Pasumarthi KB, Kardami E, Cattini PA. High and low molecular weight fibroblast growth factor-2 increase proliferation of neonatal rat cardiac myocytes but have differential effects on binucleation and nuclear morphology. Evidence for both paracrine and intracrine actions of fibroblast growth factor-2. Circ Res 1996;78(1):126-136.
Pasumarthi KB, Nakajima H, Nakajima HO, et al. Enhanced cardiomyocyte DNA synthesis during myocardial hypertrophy in mice expressing a modified TSC2 transgene. Circ Res 2000;86(10):1069-1077.
Sheikh F, Fandrich RR, Kardami E, Cattini PA. Overexpression of long or short FGFR-1 results in FGF-2-mediated proliferation in neonatal cardiac myocyte cultures. Cardiovasc Res 1999;42(3):696-705.
Gruver CL, DeMayo F, Goldstein MA, Means AR. Targeted developmental overexpression of calmodulin induces proliferative and hypertrophic growth of cardiomyocytes in transgenic mice. Endocrinology 1993;133(1):376-388.
Reiss K, Cheng W, Ferber A, et al. Overexpression of insulin-like growth factor-1 in the heart is coupled with myocyte proliferation in transgenic mice. Proc Natl Acad Sci USA 1996;93(16):8630-8635.
Miller C, Rulfs J, Jaspers SR, et al. Transformation of adult ventricular myocytes with the temperature sensitive A58 (tsA58) mutant of the SV40 large T antigen. Mol Cell Biochem 1994;136(1):29-34.
Hein L, Stevens ME, Barsh GS, et al. Overexpression of angiotensin AT1 receptor transgene in the mouse myocardium produces a lethal phenotype associated with myocyte hyperplasia and heart block. Proc Natl Acad Sci USA 1997;94(12):6391-6396.
Oh H, Taffet GE, Youker KA, et al. Telomerase reverse transcriptase promotes cardiac muscle cell proliferation, hypertrophy, and survival. Proc Natl Acad Sci USA 2001;98(18):10308-10313.
Limana F, Urbanek K, Chimenti S, et al. bcl-2 overexpression promotes myocyte proliferation. Proc Natl Acad Sci USA 2002;99(9):6257-6262.
Akli S, Zhan S, Abdellatif M, Schneider MD. E1A can provoke G1 exit that is refractory to p21 and independent of activating cdk2. Circ Res 1999;85(4):319-328.
Pajak L, Jin F, Xiao GH, et al. Sustained cardiomyocyte DNA synthesis in whole embryo cultures lacking the TSC2 gene product. Am J Physiol 1997;273(3 Pt 2):H1619-1627.
Kobayashi T, Minowa O, Kuno J, et al. Renal carcinogenesis, hepatic hemangiomatosis, and embryonic lethality caused by a germ-line Tsc2 mutation in mice. Cancer Res 1999;59(6):1206-1211.
Kirshenbaum LA, Abdellatif M, Chakraborty S, Schneider MD. Human E2F-1 reactivates cell cycle progression in ventricular myocytes and represses cardiac gene transcription. Dev Biol 1996;179(2):402-411.
Poolman RA, Li JM, Durand B, Brooks G. Altered expression of cell cycle proteins and prolonged duration of cardiac myocyte hyperplasia in p27KIP1 knockout mice. Circ Res 1999;85(2):117-127.
Shou W, Aghdasi B, Armstrong DL, et al. Cardiac defects and altered ryanodine receptor function in mice lacking FKBP12. Nature 1998;391(6666):489-492.
von Harsdorf R, Hauck L, Mehrhof F, et al. E2F-1 overexpression in cardiomyocytes induces downregulation of p21CIP1 and p27KIP1 and release of active cyclindependent kinases in the presence of insulin-like growth factor I. Circ Res 1999;85(2):128-136.
Takeuchi T, Kojima M, Nakajima K, Kondo S. jumonji gene is essential for the neurulation and cardiac development of mouse embryos with a C3H/He background. Mech Dev 1999;86(1/2):29-38.
Tamamori-Adachi M, Ito H, Sumrejkanchanakij P, et al. Critical role of cyclin D1 nuclear import in cardiomyocyte proliferation. Circ Res 2003;92(1):e12-19.
Chen F, Kook H, Milewski R, et al. Hop is an unusual homeobox gene that modulates cardiac development. Cell 2002;110(6):713-723.
Shin CH, Liu ZP, Passier R, et al. Modulation of cardiac growth and development by HOP, an unusual homeodomain protein. Cell 2002;110(6):725-735.
Bicknell KA, Brooks G. Over-expression of cyclin B1, but not CDC2, in neonatal cardiac myocytes extends their proliferative potential. Circulation 2002;106(19):II-235.
Schafer K, Neuhaus P, Kruse J, Braun T. The homeobox gene Lbx1 specifies a subpopulation of cardiac neural crest necessary for normal heart development. Circ Res 2003;92(1):73-80.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dowell, J.D., Field, L.J. & Pasumarthi, K.B. Cell Cycle Regulation to Repair the Infarcted Myocardium. Heart Fail Rev 8, 293–303 (2003). https://doi.org/10.1023/A:1024738104722
Issue Date:
DOI: https://doi.org/10.1023/A:1024738104722