Abstract
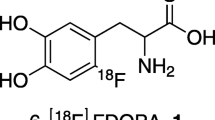
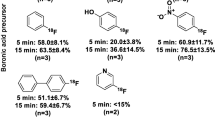
6-[18F]Fluoro-L-Dopa (6-FDOPA) is the analogue of L-Dopa, the biosynthesis precursor for dopamine. As a PET tracer, it was widely applied for the presynaptic dopamine function studies in human brain. The application of a chiral phase-transfer-catalyst (PTC) in enantioselective synthesis of N.C.A. 6-[18F]Fluoro-L-Dopa has been developed recently. An improved procedure was described in this study. The labeling precursor (6-Trimethylammoniumveratraldehyde Triflate) and PTC (O-Allyl-N-(9)-anthracenylcinchonidinium Bromide) were synthesized. A successful synthesis route was developed for the preparation of 6-[18F]Fluoro-L-Dopa with high radiochemical yields (4-9%, decay uncorrected) and short synthesis time(80min). The radiochemical purity was over 99% and no D-isomer was detected by HPLC analysis using a chiral mobile phase.
Similar content being viewed by others
References
E. J. Corey, F. Xu, M. C. Noe, J. Am. Chem. Soc., 119 (1997) 12414.
F. Dolle, S. Demphel, F. Hinnen et al., J. Label. Comp. Radiopharm., 41 (1998) 105.
G. Firnau, R. Chirakal, S. Sood et al., Can. J. Chem., 58 (1980) 1449.
D. C. Furlanno, K. L. Kirk, J. Org. Chem., 51 (1986) 4073.
E. S. Garnett, G. Firnau, C. Nahimias, Nature, 305 (1983) 137.
A. Horti, D. E. Redmond Jr., R. Soufer et al., J. Label. Comp. Radiopharm., 36 (1995) 409.
C. Lemaire, M. Guillaume, R. Cantineau et al., Appl. Radiation Isotopes, 42 (1991) 629.
C. Lemaire, M. Guillaume, R. Cantineau et al., J. Nucl. Med., 31 (1990) 1247.
C. Lemaire, A. Plenevaux, R. Cantineau et al., Appl. Radiation Isotopes, 44 (1993) 737.
C. Lemaire, P. Damhaut, A. Plenevaux et al., J. Nucl. Med., 35 (1994) 1996.
C. Lemaire, S. Guillouet, C. Brihaye et al., J. Label. Comp. Radiopharm., 42(Suppl. 1) (1999) s113.
A. Luxen, G. T. Bida, M. E. Phelps et al., J. Nucl. Med., 28 (1987) 624.
A. Luxen, M. Guillaume, W. P. Melega et al., Nucl. Med. Biol., 19 (1992) 149.
A. Luxen, M. Perlmutter, G. T. Bida et al., Appl. Radiation Isotopes, 41 (1990) 275.
A. Najafi, Nucl. Med. Biol., 22 (1995) 395.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yin, D., Zhang, L., Tang, G. et al. Enantioselective synthesis of no-carrier added (NCA) 6-[18F]Fluoro-L-Dopa. Journal of Radioanalytical and Nuclear Chemistry 257, 179–185 (2003). https://doi.org/10.1023/A:1024734402290
Issue Date:
DOI: https://doi.org/10.1023/A:1024734402290