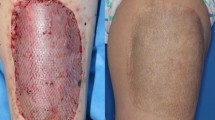
Abstract
Skin defects left after excision of hypertrophic scars were treated with a dermal substitute and split-thickness skin grafts transplanted after vascularisation of the substitute. The used substitute was a synthetic porous scaffold made from the biodegradable copolymer polyethyleneglycol-terephtalate and polybuthylene-terephtalate. The study was designed to assess the rate of granulation tissue formation, graft take, and after 3 and 12 months the quality of life (pain, comfort of treatment, cosmetic or functional nuisance), scar formation and wound contraction. In addition, scaffold biodegradation and scar tissue formation were evaluated histologically.
Seven patients with different causes of burn injury were enrolled, of which 5 completed the study. In the first 4 patients the time between scaffold application and split-thickness skin overgrafting was in between 17 and 24 days. The time point of overgrafting was significantly reduced to 10–12 days by meshing of the dermal scaffold as evidenced in the last 3 patients. Histological evaluation at 3 months revealed normal generation of dermal tissue, however, the collagen bundles were parallel organized like in scar tissue. In the deeper layers of the neodermis, fragments of the dermal substitute were present, causing a mild inflammatory response. One year post-treatment, some fragments of the copolymer were still observed. The extent of wound contraction after successful overgrafting ranged from 30% to 57% after 1 year. All 5 patients showed an improvement in the total Vancouver Scar Score compared to the value before scar removal being similar to what can be expected when treated with split-thickness skin grafts alone. No unanticipated adverse effects due to application of the substitute were observed.
We conclude that although this synthetic dermal substitute can be safely used in humans, the presence of 3D dermal template in a full-thickness skin defect will not automatically improve the skin tissue regeneration process or inhibit wound contraction.
Similar content being viewed by others
References
Beumer GJ, van Blitterswijk CA, Bakker D and Ponec M (1993a) Cell-seeding and in vitro biocompatibility evaluation of polymeric matrices of PEO/PBT copolymers and PLLA. Biomaterials 14: 598–604
Beumer GJ, van Blitterswijk CA, Bakker D and Ponec M (1993b) A new biodegradable matrix as part of a cell seeded skin substitute for the treatment of deep skin defects: A physico-chemical characterisation. Clin Mater 14: 21–27
Beumer GJ, van Blitterswijk CA and Ponec M (1994a) Biocompatibility of a biodegradable matrix used as a skin substitute: An in vivo evaluation. J Biomed Mater Res 28: 545–552
Beumer GJ, van Blitterswijk CA and Ponec M (1994b) Degradative behaviour of polymeric matrices in (sub)dermal and muscle tissue of the rat: A quantitative study. Biomaterials 15: 551–559
Boyce ST, Warden GD and Holder IA (1995a) Non-cytotoxic combinations of topical antimicrobials agents for use with cultured skin. Antimicrob Agents Chemother 39: 1324–1328
Boyce ST, Goretsky MJ, Greenhalgh DG, Kagan RJ, Rieman MT and Warden GD (1995b) Comparative assessment of cultured skin substitutes and native skin autograft for treatment of full-thickness burns. Ann Surg 22: 743–752
Burke JF, Yannas IV, Quinby WC Jr, Bondoc CC and Jung WK (1981) Successful use of a physiologically acceptable artificial skin in the treatment of extensive burn injury. Ann Surg 194: 413–428
Cooper ML, Hansbrough JF, Spielvogel RL, Cohen R, Bartel RL and Naughton G (1991) In vivo optimization of a living dermal substitute employing cultured human fibroblasts on a biodegradable polyglycolic acid or polyglactin mesh. Biomaterials 12: 243–248
Cuono C, Langdon R and McGuire J (1986) Use of cultured epidermal autografts and dermal allografts as skin replacement after burn injury. Lancet 17(1): 1123–1124
De Vries HJ, Zeegelaar JE, Middelkoop E, et al. (1995a) Reduced wound contraction and scar formation in punch biopsy wounds. Native collagen dermal substitutes, a clinical study. Br J Dermatol 132: 690–697
De Vries HJ, Middelkoop E, van Heemstra-Hoen M, Wildevuur CH and Westerhof W (1995b) Stromal cells from subcutaneous adipose tissue seeded in a native collagen/elastin dermal substitute reducewound contraction in full thickness skin defects. Lab Invest 73: 532–540
El Hadidy M, Tesauro P, Cavallini M, Colonna M, Rizzo F and Signorini M (1994) Contraction and growth of deep burn wounds covered by non-meshed and meshed split thickness skin grafts in humans. Burns 20: 226–228
Hansbrough JF, Dore C and Hansbrough WB (1992) Clinical trials of a living dermal tissue replacement placed beneath meshed, split-thickness skin grafts on excised burn wounds. J Burn Care Rehabil 13: 519–529
Hansbrough JF, Morgan JL, Greenleaf GE and Bartel R (1993) Composite grafts of human keratinocytes grown on a polyglactin mesh-cultured fibroblast dermal substitute function as a bilayer skin replacement in full-thickness wounds on athymic mice. J Burn Care Rehabil 14: 485–494
Heimbach D, Luterman A, Burke J, et al. (1988) Artificial dermis for major burns. A multi-center randomized clinical trial. Ann Surg 208: 313–320
Lamme EN, Van Leeuwen RT, Brandsma K, Van Marle J and Middelkoop E (2000) Higher numbers of autologous fibroblasts in an artificial dermal substitute improve tissue regeneration and modulate scar tissue formation. J Pathol 190: 595–603
Lamme EN, van Leeuwen RT, Jonker A, van Marle J and Middelkoop E (1998) Living skin substitutes: Survival and function of fibroblasts seeded in a dermal substitute in experimental wounds. J Invest Dermatol 111: 989–995
Moiemen NS, Staiano JJ, Ojeh NO, Thway Y and Frame JD (2001) Reconstructive surgery with a dermal regeneration template: Clinical and histologic study. Plast Reconstr Surg 108: 93–103
Mortelmans K, Haworth S, Lawlor T, Speck W, Tainer B and Zeiger E (1986) Salmonella mutagenicity tests: II. Results from the testing of 270 chemicals. Environ Mutagen 8(Suppl 7): 1–119
Nave M (1992) Wound bed preparations: Approaches to replacement of dermis. J Burn Care Rehabil 13: 147–153
Nie L, Nicolau DP, Nightingale CH, Browner BD and Quintiliani R (1995) In vitro elution of ofloxacin from a bioabsorbable polymer. Acta Orthop Scand 66: 365–368
Press B (1997) Thermal, electrical, and chemical injuries. In: Sherrell JA, Beasley RW and Thorne CHM (eds) Grabb and Smith's Plastic Surgery, 5th edition, pp 161–189. Lippincott- Raven Publishers, Philadelphia
Radder AM, Davies JE, Leenders H and van Blitterswijk CA (1994a) Interfacial behavior of PEO/PBT copolymers (Polyactive) in a calvarial system: An in vitro study. J Biomed Mater Res 28: 269–277
Radder AM, Leenders H and van Blitterswijk CA (1994b) Interface reactions to PEO/PBT copolymers (Polyactive) after implantation in cortical bone. J Biomed Mater Res 28: 141–151
Radder AM, Leenders H and van Blitterswijk CA (1996) Application of porous PEO/PBT copolymers for bone replacement. J Biomed Mater Res 30: 341–351
Sclafani AP, Romo T 3rd, Jacono AA, McCormick SA, Cocker R and Parker A (2001) Evaluation of acellular dermal graft (AlloDerm) sheet for soft tissue augmentation: A 1-year follow-up of clinical observations and histological findings. Arch Facial Plast Surg 3: 101–103
Stern R, McPherson M and Longaker MT (1990) Histologic study of artificial skin used in the treatment of full thickness thermal injury. J Burn Care Rehabil 11: 7–13
Sullivan T, Smith J, Kermode J, McIver E and Courtemanche DJ (1990) Rating the burn scar. J Burn Care Rehabil 11: 256–260
Van Dorp AG, Verhoeven MC, Koerten HK, Van Der Nat-Van Der Meij TH, Van Blitterswijk CA and Ponec M (1998) Dermal regeneration in full-thickness wounds in Yucatan miniature pigs using a biodegradable copolymer. Wound Repair Regen 6: 556–568
Van Dorp AG, Verhoeven MC, Koerten HK, van Blitterswijk CA and Ponec M (1999) Bilayered biodegradable poly(ethylene glycol)/poly(butylene terephthalate) copolymer (Polyactive) as substrate for human fibroblasts and keratinocytes. J Biomed Mater Res 47: 292–300
Van Zuijlen PP, van Trier AJ, Vloemans JF, Groeneveldt F, Kreis RW and Middelkoop E (2000) Graft survival and effectiveness of dermal substitution in burns and reconstructive surgery in a one-stage grafting model. Plast Reconstr Surg 106: 615–623
Wainwright DJ (1995) Use of an acellular allograft dermal matrix (AlloDerm) in the management of full-thickness burns. Burns 21: 243–248
Yannas IV, Burke JF, Gordon PL, Huang C and Rubenstein RH (1980) Design of an artificial skin. II. Control of chemical composition. J Biomed Mater Res 14: 107–132
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mensik, I., Lamme, E., Riesle, J. et al. Effectiveness and Safety of the PEGT/PBT Copolymer Scaffold as Dermal Substitute in Scar Reconstruction Wounds (Feasibility Trial). Cell Tissue Banking 3, 245–253 (2002). https://doi.org/10.1023/A:1024674325253
Issue Date:
DOI: https://doi.org/10.1023/A:1024674325253