Abstract
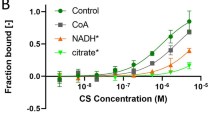
Steady state kinetics of bovine heart NADH: coenzyme Q oxidoreductase using coenzyme Q with two isoprenoid unit (Q2) or with a decyl group (DQ) show an ordered sequential mechanism in which the order of substrate binding and product release is NADH-Q2 (DQ) -Q2H2 (DQH2)-NAD+ in contrast to the order determined using Q1 (Q1-NADH-NAD+-Q1H2) (Nakashima et al., J. Bioenerg. Biomembr. 34, 11–19, 2002). The effect of the side chain structure of coenzyme Q suggests that NADH binding to the enzyme results in a conformational change, in the coenzyme Q binding site, which enables the site to accept coenzyme Q with a side chain significantly larger than one isoprenoid unit. The side chains of Q2 and DQ bound to the enzyme induce a conformational change in the binding site to stabilize the substrate binding, while the side chain of Q1 (one isoprenoid unit) is too short to induce the conformational change.
Similar content being viewed by others
References
Buchanan, S. K., and Walker, J. E. (1996). Biochem. J. 318, 343–349.
Cleland, W. W. (1977). Adv. Enzymol. 45, 273–387.
Hatefi, Y., Haavik, A. G., and Jurtshuk, P. (1961). Biochim. Biophys. Acta 52, 106–118.
Hatefi, Y. (1985). Ann. Rev. Biochem. 54, 1015–1069.
Koshland, D. E. Jr. (1973). Sci. Am. 229, 52–64.
Nakashima, Y., Shinzawa-Itoh, K., Watanabe, K., Naoki, K., Hano, N., and Yoshikawa, S. (2002a). J. Bioenerg. Biomembr. 34, 11–19.
Nakashima, Y., Shinzawa-Itoh, K., Watanabe, K., Naoki, K., Hano, N., and Yoshikawa, S. (2002b). J. Bioenerg. Biomembr. 34, 89–94.
Ohnishi, T. (1998). Biochim. Biophys. Acta 1364, 186–206.
Rieske, J. S. (1967). Methods Enzymol. 10, 239–245.
Tamura, S., Takahashi, N., Miyamoto, S., Mori, R., Suzuki, S., and Nagatsu, J. (1963). Agric. Biol. Chem. 27, 576–582.
Walker, J. E. (1992). Q. Rev. Biophys. 25, 253–324.
Weiss, H., Friedrich, T., Hofhaus, G., and Preis, D. (1991). Eur. J. Biochem. 197, 563–576.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hano, N., Nakashima, Y., Shinzawa-Itoh, K. et al. Effect of the Side Chain Structure of Coenzyme Q on the Steady State Kinetics of Bovine Heart NADH: Coenzyme Q Oxidoreductase. J Bioenerg Biomembr 35, 257–265 (2003). https://doi.org/10.1023/A:1024663715931
Issue Date:
DOI: https://doi.org/10.1023/A:1024663715931