Abstract
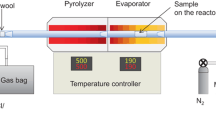
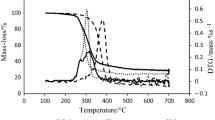
The thermochemical behaviour of sugars (D- and DL-arabinose, D- and DL-xylose and D-mannose) and sugar alcohol (D- and DL-arabinitol) was investigated by TG and pyrolysis-gas chromatography with mass-selective detection (Py-GC/MSD). The temperature of pyrolysis was 500 and 550°C. The TG-curves were measured both in air and nitrogen atmospheres, from 25 to 700°C with the heating rate of 2°C min-1. In each case, the main pyrolysis products were classified into the following compound groups: (i) furanes, (ii) pyranes, (iii) cyclopentanes, (iv) cyclohexanes, (v) anhydroglucopyranoses, (vi) dianhydroglucopyranoses and (vii) saturated fatty acids. For example, the main peaks of the chromatograms of pentoses (arabinose, xylose), hexose (mannose) and sugar alcohols (arabinitols) were different. The greatest peak of pentoses in gas-chromatogram was 2-furancarboxaldehyde and that of hexose was (2H)-furan-3-one. The greatest peak of arabinitols at pyrolysis temperature of 500°C was furan methanol and at 550°C a-angeligalactone. 5-hydroxymethyl-2-furan carboxaldehyde was found only in the pyrolysis of D-mannose (hexose). The former study showed that it was not found in pyrolysis of pentoses. The amount of CO2 and H2O was not determined.
Similar content being viewed by others
References
O. Hassel and B. Ottar, Acta Chem. Scand., 1 (1974) 929.
R. E. Reeves, J. Am. Chem. Soc., 72 (1950) 1499.
P. R. Sundararajan and V. S. Rao, Tedrahedron, 24 (1968) 289.
D. Meier and O. Faix, Eds. S. Y. Lin and C. W. Dence, Methods in Lignin Chemistry, Springer-Verlag, Berlin 1992, pp. 177-199.
W. Xie and W.-P. Pan, J. Therm. Anal. Cal., 65 (2001) 669.
P. Linko, T. Saijonmaa, M. Heikonen and M. Kreula, Carbohydr. Sweeteners Foods Nutr., (1980) 243.
I. Pitkänen, J. Huttunen, H. Halttunen and R. Vesterinen, J. Therm. Anal. Cal., 56 (1999) 1253.
J. Suuronen, I. Pitkänen, H. Halttunen and R. Moilanen, J. Therm. Anal. Cal., 69 (2002) 359.
H. Halttunen, I. Pitkänen and U. Räisänen, J. Anal. Appl. Pyrolysis, in press.
R. Alén, E. Kuoppala and P. Oesch, J. Anal. Appl. Pyrolysis, 36 (1996) 137.
Y. Roos, Carbohydr. Res., 238 (1993) 39.
A. Raemy and T. F. Schweizer, J. Thermal Anal., 28 (1983) 95.
L. Slade and H. Levine, Crit. Rev. Food Sci. Nutr., 30 (1991) 115.
C. Ramos-Sanchez, F. J. Rey, L. Rodriquez-Mendez, F. J. Martin-Gil and J. Martin-Gil, Thermochim. Acta, 134 (1988) 55.
Y. Kaburaki, U. Kobashi, T. Doihara and S. Sugawara, Nippon Sembai Kosha Chuo Kenkyusho Kenkyu Hokoku, 108 (1966) 355, Chem. Abstr., 66 (1967) 18818v.
A. Ohnishi, K. Kato and E. Takagi, Carbohydr. Res., 58 (1977) 387.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Räisänen, U., Pitkänen, I., Halttunen, H. et al. Formation of the main degradation compounds from arabinose, xylose, mannose and arabinitol during pyrolysis. Journal of Thermal Analysis and Calorimetry 72, 481–488 (2003). https://doi.org/10.1023/A:1024557011975
Issue Date:
DOI: https://doi.org/10.1023/A:1024557011975