Abstract
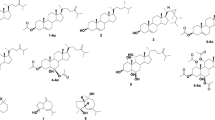
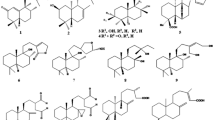
The structures of products obtained by reductive debromination and CF3COOH- and KOH-induced transformations of natural chamigrane-type sesquiterpenoid (6S,10R)-10-bromo-3,11,11-trimethyl-7-methylidenespiro[5,5]undec-2-en-4-one (dactylone) isolated from the sea hare Aplysia dactylomela were analyzed. The absolute configurations of the reaction products were established by CD spectra taking into account the configuration of the starting dactylone.
Similar content being viewed by others
References
G. B. Elyakov and V. A. Stonik, Terpenoidy morskikh organizmov [Terpenoids of Marine Organisms], Nauka, Moscow, 1986, 271 pp. (in Russian).
S. N. Fedorov, O. S. Radchenko, L. K. Shubina, A. I. Kalinovsky, A. V. Gerasimenko, D. Y. Popov, and V. A. Stonik, J. Am. Chem. Soc., 2001, 123, 504.
D. J. Faulkner, Nat. Prod. Rep., 1995, 12, 223.
D. J. Faulkner, Nat. Prod. Rep., 1996, 13, 75.
G. M. Konig and A. D. Wright, J. Nat. Prod., 1997, 60, 967.
K. A. El Sayed, D. C. Dunbar, T. L. Perry, S. P. Wilkins, and M. T. Hamman, J. Agric Food Chem., 1997, 45, 2735.
E. G. Juagdun, R. Kalidindi, and P. J. Scheuer, Tetrahedron, 1997, 53, 521.
S. N. Fedorov, M. V. Reshetnyak, A. P. Shchedrin, S. G. Il´in, Yu. T. Struchkov, V. A. Stonik, and G. B. Elyakov, Dokl. Akad. Nauk SSSR, 1989, 305, 877 [Dokl. Chem., 1989 (Engl. Transl.)].
S. N. Fedorov, L. K. Shubina, A. I. Kalinovsky, E. G. Lyakhova, and V. A. Stonik, Tetrahedron Lett., 2000, 41, 1979.
B. Lacoume, Bull. Soc. Chim. Fr., 1967, 3496.
N. S. Reddy, T. V. Goud, and Y. Venkateswarlu, J. Chem. Res. S, 2000, 9, 438.
F. J. McEnroe and W. Fenicial, Tetrahedron, 1978, 34, 1661.
C. Y. Chen, Y. C. Shen, Y. J. Chen, J. H. Sheu, and C. Y. Duh, J. Nat. Prod., 1999, 62, 573.
L. Fieser and M. Fieser, Reagents for Organic Synthesis, New York, 1967, 1, 1276.
Organic Synthesis, Ed. H. Gilman, Quins College, Flushing, New York, 1944.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lyakhova, E.G., Fedorov, S.N., Shubina, L.K. et al. Chemical properties of marine terpenoids. 1. Some reactions of (6S,10R)-10-bromo-3,11,11-trimethyl-7-methylidenespiro[5,5]undec-2-en-4-one, a sesquiterpenoid from the sea hare Aplysia dactylomela . Russian Chemical Bulletin 52, 1022–1026 (2003). https://doi.org/10.1023/A:1024489418232
Issue Date:
DOI: https://doi.org/10.1023/A:1024489418232