Abstract
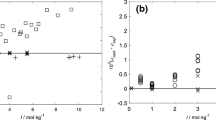
Dilution enthalpies, measured using isothermal flow calorimetry, are reported for aqueous solutions of LiCl, KCl, and CsCl at 300°C and 11.0 MPa, 325°C and 14.8 MPa, and 350°C and 17.6 MPa. The concentration range of the chloride solutions was 0.5 to 0.02 m. Parameters for the Pitzer excess Gibbs ion-interaction equation were determined from the fits of the experimental heat data. Equilibrium constants, enthalpy changes, entropy changes, and heat capacity changes for ion association of the chloride salts were estimated from the heat data. For all systems, the enthalpy and entropy changes were positive and had accelerating increases with temperature. The resulting equilibrium constants show significant, but smaller, increases with temperature.
Similar content being viewed by others
REFERENCES
J. L. Oscarson, R. M. Izatt, P. R. Brown, Z. Pawlak, S. E. Gillespie, and J. J. Christensen, J. Solution Chem. 17, 841 (1988).
J. L. Oscarson, S. E. Gillespie, J. J. Christensen, R. M. Izatt, and P. R. Brown, J. Solution Chem. 17, 865 (1988).
J. L. Oscarson, S. E. Gillespie, R. M. Izatt, X. Chen, and C. Pando, J. Solution Chem. 21, 761 (1992).
S. E. Gillespie, J. L. Oscarson, X. Chen, R. M. Izatt, and C. Pando, J. Solution Chem. 21, 789 (1992).
X. Chen, S. E. Gillespie, J. L. Oscarson, and R. M. Izatt, J. Solution Chem. 21, 825 (1992).
X. Chen, S. E. Gillespie, J. L. Oscarson, and R. M. Izatt, J. Solution Chem. 21, 803 (1992).
R. M. Izatt, J. L. Oscarson, S. E. Gillespie, X. Chen, P. Wang, and G. D. Watt, Pure Appl. Chem. 67, 543 (1995).
X. Chen, J. L. Oscarson, S. E. Gillespie, H. Cao, and R. M. Izatt, J. Solution Chem. 23, 747 (1994).
R. M. Izatt, S. E. Gillespie, X. Chen, and J. L. Oscarson, Pure Appl. Chem. 63, 1419 (1991).
X. Chen, R. M. Izatt, and J. L. Oscarson, Chem. Rev. 94, 467 (1994).
J. L. Oscarson, X. Chen, S. E. Gillespie, and R. M. Izatt, Thermochim. Acta 185, 51 (1991).
S. E. Gillespie, J. L. Oscarson, R. M. Izatt, P. Wang, J. A. R. Renuncio, and C. Pando. J. Solution Chem. 24, 1221 (1995).
D. G. Archer, J. Phys. Chem. Ref. Data 21, 793 (1992).
X. Chen, J. L. Oscarson, H. Cao, S. E. Gillespie, and R. M. Izatt, Thermochim. Acta 285, 11 (1996).
R. M. Izatt, J. L. Oscarson, S. E. Gillespie, and X. Chen, Determination of Thermodynamic Data for Modeling Corrosion, Volume 4: Chloride Ion Interaction with Magnesium, Calcium, and Hydrogen Ions at 250, 275, 300 and 325°C, EPRI Report NP-5708 (Electric Power Research Institute, Palo Alto, CA, 1992).
G. A. Gates and R. H. Wood, J. Chem. Eng. Data 30, 44 (1985).
I. D. Zaytsev, G. G. Aseyev eds. Properties of Aqueous Solutions of Electrolytes, M. A. and V. R. Sorochenko (translators) (CRC Press, Boca Raton, FL, 1992).
K. S. Pitzer, J. C. Peiper, and R. H. Busey, J. Phys. Chem. Ref. Data 13, 1 (1984).
R. H. Busey, H. F. Holmes, and R. E. Mesmer, J. Chem. Thermodyn. 16, 1 (1984).
R. M. Izatt, S. E. Gillespie, J. L. Oscarson, P. Wang, J. A. R. Renuncio, and C. Pando, J. Solution Chem. 23, 449 (1994).
J. M. Simonson, H. F. Holmes, R. H. Busey, R. E. Mesmer, D. G. Archer, and R. H. Wood, J. Phys. Chem. 94, 7675 (1990).
H. F. Holmes, R. H. Busey, J. M. Simonson, R. E. Mesmer, D. G. Archer, and R. H. Wood, J. Chem. Thermodyn. 19, 863 (1987).
K. S. Pitzer, in Activity Coefficients in Electrolyte Solutions, 2nd Edition, K. S. Pitzer, ed. (CRC Press, Boca Raton, FL, 1991).
L. Haar, J. S. Gallagher, and G. S. Kell, NBS/NRC Steam Tables. Thermodynamic and Transport Properties and Computer Programs for Vapor and Liquid States of Water in SI Units. (Hemisphere, Washington, 1984).
G. H. Zimmerman, M. S. Gruszkiewicz, and R. H. Wood, J. Phys. Chem. 99, 11612 (1995).
A. S. Quist and W. L. Marshall, J. Phys. Chem. 73, 987 (1969).
E. H. Oelkers and H. C. Helgeson, J. Phys. Chem. 92, 1631 (1988).
J. M. Simonson, R. H. Busey, and R. E. Mesmer, J. Phys. Chem. 89, 557 (1985).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gillespie, S.E., Chen, X., Oscarson, J.L. et al. Enthalpies of Dilution of Aqueous Solutions of LiCl, KCl, and CsCl at 300, 325, and 350°C. Journal of Solution Chemistry 26, 47–61 (1997). https://doi.org/10.1023/A:1024485028264
Issue Date:
DOI: https://doi.org/10.1023/A:1024485028264