Abstract
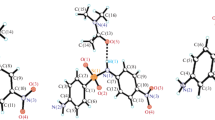
Crystalline specimens of homochiral and racemic glycidyl p-toluenesulfonate were studied by IR spectroscopy, differential scanning calorimetry, and X-ray diffraction analysis. The melting phase diagram of glycidyl p-toluenesulfonate was constructed. The stacking effect in the crystals of the racemic sulfonate is responsible for a more dense molecular packing, with the result that a heterochiral type of crystallization becomes more favorable.
Similar content being viewed by others
References
J. Jaques, A. Collet, and S. H. Wilen, Enantiomers, Racemates, and Resolutions, Krieger Publishing Co., Malabar, FL, 1994, 447 pp.
Z. Bocskei, C. Kassai, K. Simon, E. Fogassy, and D. Kozma, J. Chem. Soc., Perkin Trans. 2, 1996, 1511.
K. Kinbara, Y. Hashimoto, M. Sukegawa, H. Nohira, and K. Saigo, J. Am. Chem. Soc., 1996, 118, 3441.
S. M. Reutzel-Edens, V. A. Russell, and L. Yu, J. Chem. Soc., Perkin. Trans. 2, 2000, 913.
G. Muller and M. Lutz, Z. Naturforsch., 2001, 56B, 871.
R. G. Kostyanovsky, V. R. Kostyanovsky, G. K. Kadorkina, and K. A. Lyssenko, Mendeleev Commun., 2001, 1.
C. P. Brock, W. B. Schweizer, and J. D. Dunitz, J. Am. Chem. Soc., 1991, 113, 9811.
A. Gavezzotti, Acc. Chem. Res., 1994, 27, 309.
J. Hulliger, Angew. Chem., Int. Ed. Engl., 1994, 33, 143.
S. H. Wilen, A. Collet, and J. Jacques, Tetrahedron, 1977, 33, 2725.
A. Collet, J. Jacques, and M. J. Brienne, Chem. Rev., 1980, 80, 215.
R. M. Hanson, Chem. Rev., 1991, 91, 437.
Beilst., EV, 17(1), 9.
J. M. Klunder, T. Onami, and K.B. Sharpless, J. Org. Chem., 1989, 54, 1295.
Y. Gao, R. M. Hanson, J. M. Klunder, S. Y. Ko, H. Masamune, and K. B. Sharpless, J. Am. Chem. Soc., 1987, 109, 5765.
N. Nakabayashi, E. Masuhara, and Y. Iwakura, Bull. Chem. Soc. Jpn., 1966, 39, 413.
A. Altomare, G. Cascarano, C. Giacovazzo, and D. Viterbo, Acta Crystallogr., Sect. A, 1991, 47, 744.
W. C. Hamilton, Acta Crystallogr., 1965, 18, 502.
L. H. Straver and A. J. Schierbeek, MolEN, Structure Determination System, Program Description, Nonius B.V., Delft, 1994, 1, 180.
A. L. Spek, Acta Crystallogr., Sect A, 1990, A46, 34.
E. L. Eliel, S. H. Wilen, and L. N. Mander, Stereochemistry of Organic Compounds, John Wiley and Sons, New York, 1994, p. 297; E. L. Eliel, S. H. Wilen, and M. P. Doyle, Basic Organic Stereochemistry, Wiley-Interscience, New York, 2001, p. 197.
C. P. Brock and J. D. Dunitz, Chem. Mater., 1994, 6, 1118.
A. I. Kitaigorodsky, Molecular Crystals and Molecules, Academic Press, New York-London, 1973.
M. C. Etter, Acc. Chem. Res., 1990, 23, 120.
J. Bernstein, R. E. Davis, L. Simoni, and N. L. Chang, Angew. Chem., Int. Ed. Engl., 1995, 34, 1555.
T. Dahl, Acta Chem. Scand., 1994, 48, 95.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bredikhin, A.A., Lazarev, S.N., Bredikhina, Z.A. et al. Crystallization of chiral compounds. 1. Spectroscopic, thermochemical, and crystallographic investigation of homochiral and racemic glycidyl p-toluenesulfonate. Russian Chemical Bulletin 52, 846–852 (2003). https://doi.org/10.1023/A:1024483822350
Issue Date:
DOI: https://doi.org/10.1023/A:1024483822350