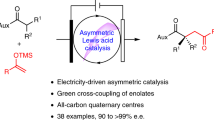
Abstract
Electrolysis of dialkyl ketones in MeOH in the presence of the NaI—NaOH mediator system placed in an undivided cell involves a process analogous to the Favorsky rearrangement of α,α-dihalodialkyl ketones giving rise to methyl esters of α,β-unsaturated carboxylic acids in 70—75% substance yields and 60—70% current yields.
Similar content being viewed by others
References
S. K. Chakrabartty, in Oxidation in Organic Chemistry, Pt. C, Ed. W. S. Trahanovsky, Academic Press, New York, 1978, p. 343.
R. T. Arnold, R. Buckles, and J. Stoltenberg, J. Am. Chem. Soc., 1944, 66, 208.
R. Levine and J. R. Stephens, J. Am. Chem. Soc., 1950, 72, 1642.
R. C. Fuson and B. A. Bull, Chem. Rev., 1934, 15, 275.
J. Grimshow, Electrochemical Reactions and Mechanisms in Organic Chemistry, Elsevier, Amsterdam, 2000.
Organic Electrochemistry, Ed. H. Lund, Marcel Dekker, Inc., New York, 2000.
W. E. Bradt and N. J. Opp, Trans. Electrochem. Soc., 1931, 59, 237.
M. Yokoyama, Bull. Chem. Soc. Jpn., 1933, 8, 71.
F. Pirrone, Gazz. Chim. Ital., 1936, 66, 244.
J. W. Shipley and M. T. Rogers, Can. J. Res., 1939, 17B, 147.
J. Y. Becker, L. R. Byrd, L. L. Miller, and Y.-H. So, J. Am. Chem. Soc., 1975, 97 , 853.
C. B. Campbell and D. Pletcher, Electrochim. Acta, 1978, 23, 953.
C. Rappe, in The Chemistry of the Carbon-Halogen Bond, Ed. S. Patai, Wiley, New York, 1973, Pt. 2, p. 1071.
G. I. Nikishin, M. N. Elinson, and I. V. Makhova, Angew. Chem., 1988, 100, 1716.
M. N. Elinson, I. V. Makhova, and G. I. Nikishin, Tetrahedron, 1991, 47, 895.
T. Shono, Y. Matsumura, K. Inoe, and F. Iwasaki, J. Chem. Soc., Perkin Trans. 1, 1986, 73.
F. Barba, M. N. Elinson, J. Escudero, and S. K. Feducovich, Tetrahedron, 1997, 53, 4427.
M. N. Elinson, S. K. Feducovich, A. S. Dorofeev, A. N. Vereshchagin, and G. I. Nikishin, Tetrahedron, 2000, 56, 9999.
F. Barba, M. N. Elinson, J. Escudero, M. Guirado, and S. K. Feducovich, Electrochim. Acta, 1998, 43 , 973.
H. C. Brown, J. Chem. Soc., 1956, 1248.
H. O. Krabbenhoft, J. Org. Chem., 1979, 44, 4285.
J. Palaty and F. S. Abbott, J. Med. Chem., 1995, 38, 3406.
S. Derien, E. Dunach, and J. Perichon, J. Am. Chem. Soc., 1991, 113; 8447.
T. K. Chumachenko, G. L. Kamalov, A. V. Bogatskii, and A. I. Gren´, Zh. Org. Khim., 1970, 40, 846 [J. Org. Chem. USSR, 1970, 40 (Engl. Transl.)].
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Elinson, M.N., Feducovich, S.K., Zaimovskaya, T.A. et al. Electrochemically induced Favorsky rearrangement: transformations of dialkyl ketones into α,β-unsaturated carboxylic esters. Russian Chemical Bulletin 52, 998–1002 (2003). https://doi.org/10.1023/A:1024481216415
Issue Date:
DOI: https://doi.org/10.1023/A:1024481216415