Abstract
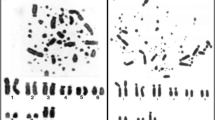
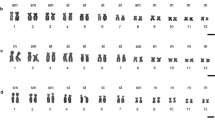
The Japanese rose bitterling, Rhodeus ocellatus kurumeus, and the oily bitterling, Tanakia limbata, were cytogenetically studied by silver (Ag)- and chromomycin A3 (CMA3)-staining, by C-banding and by mapping of the 18S ribosomal genes and of the (TTAGGG) n telomeric sequence. These two representative species of related genera of the subfamily Acheilognathinae show very similar chromosome complements. Nevertheless, significant differences in the chromosomal distribution of nucleolus organizer regions (NORs) and interstitial telomeric sequences were observed. Whereas R. ocellatus kurumeus shows a single NOR-bearing chromosome pair, T. limbata is characterized by a higher number of variable NORs. Multiple telomeric sequence sites were found at the pericentromeric regions of several chromosomes in the rose bitterling. No telomeric sequence sites were detected near centromeres, but they were found to be scattered along the NORs in the oily bitterling. Two karyoevolutive trends might have been identified in the subfamily.
Similar content being viewed by others
References
Arai, R. & Y. Akai, 1988. Acheilognathus melanogaster, a senior synonym of A. moriokae, with a revision of the genera of the subfamily Acheilognathinae (Cypriniformes, Cyprinidae). Bull. Natl. Sci. Mus. Tokyo, (A) 14: 199–213.
Ashley, T. & D.C. Ward, 1993. A 'hot spot' of recombination coincides with an interstitial telomeric sequence in the Armenian hamster. Cytogenet. Cell Genet. 62: 169–171.
Caputo, V., M. Sorice, R. Vitturi, R. Magistrelli & E. Olmo, 1998. Cytogenetic studies in some species of Scorpaeniformes (Teleostei: Percomorpha). Chromosome Res. 6: 255–262.
Chew, J.S.K., C. Oliveira, J.M. Wright & M.J. Dobson, 2002. Molecular and cytogenetic analysis of the telomeric (TTAGGG)n repetitive sequences in the Nile tilapia, Oreochromis niloticus (Teleostei: Cichlidae). Chromosoma 111: 45–52.
Fontana, F., M. Lanfredi, M. Chicca, V. Aiello & R. Rossi, 1998. Localization of the repetitive telomeric sequence (TTAGGG)n in four sturgeon species. Chromosome Res. 6: 303–306.
Gornung, E.M., I. Gabrielli & L. Sola, 1998. Localization of the (TTAGGG)n telomeric sequences in zebrafish chromosomes. Genome 41: 136–138.
Howell, W.M. & D.A. Black, 1980. Controlled silver-staining of nucleolus organizer regions with a protective colloidal developer: a 1–step method. Experientia 36: 1014–1015.
John, B. & G.L.G. Miklos, 1979. Functional aspects of satellite DNA and heterochromatin. Int. Rev. Cytol. 58: 1–114.
Kawamura, K., T. Ueda, R. Arai, Y. Nagata, K. Saitoh, H. Ohtaka & Y. Kanoh, 2001. Genetic introgression by the rose bitterling, Rhodeus ocellatus ocellatus, into the Japanese rose bitterling, R. ocellatus kurumeus (Teleostei: Cyprinidae). Zool. Sci. 18: 1027–1039.
Kilburn, A.E., M.J. Shea, R.G. Sargent & J.H. Wilson, 2001. Insertion of a telomere repeat sequences into a mammalian gene causes chromosome instability. Mol. Cell Biol. 21: 126–135.
Lichter, P., A. Boyle, J. Wienberg, N. Arnold, S. Popp, T. Cremer & D.C. Ward, 1992. In situ hybridization to human metaphase chromosomes using DIG-or biotin-labeled DNA probes and detection with fluorochrome conjugates, pp. 25–27 in Nonradioactive In situ Hybridization (Application Manual). Boehringer Mannheim, Biochemica, Mannheim.
Mandrioli, M. & G.C. Manicardi, 2001. Cytogenetic and molecular analysis of the pufferfish Tetraodon fluviatilis (Osteichthyes). Genetica 111: 433–438.
Meyne, J., R.L. Ratliff & R.K. Moyzis, 1989. Conservation of the human telomere sequence (TTAGGG)n among vertebrates. Proc. Natl. Acad. Sci. USA 89: 7049–7053.
Meyne, J., R.J. Baker, H.H. Hobart, T.C. Hsu, O.A. Ryder, O.G. Ward, J.E. Wiley, D.H. Wurster-Hill, T.L. Yates & R.K. Moyzis, 1990. Distribution of non-telomeric sites of the (TTAGGG)n telomeric sequences in vertebrate chromosomes. Chromosoma 99: 3–10.
Meyne, J., H. Hirai & H.T. Imai, 1995. FISH analysis of the telomere sequences of bulldog ants (Myrmecia: Formicidae). Chromosoma 104: 14–18.
Ojima, Y., K. Ueno & M. Hayashi, 1973. Karyotypes of the acheilognathine fishes (Cyprinidae) of Japan with a discussion of phylogenetic problems. Zool. Mag. (Tokyo) 82: 171–177 (in Japanese with English abstract).
Okazaki, M., K. Naruse, A. Shima & R. Arai, 2001. Phylogenetic relationships of bitterlings based on mitochondrial 12S ribosomal DNA sequences. J. Fish Biol. 58: 89–106.
Pagnozzi, J.M., M.J.D. Silva & Y. Yonenaga-Yassuda, 2000. Intraspecific variation in the distribution of the interstitial telomeric (TTAGGG)n sequences in Micoureus demerarae (Marsupialia: Didelphidae). Chromosome Res. 8: 585–591.
Reed, K.M. & R.B. Phillips, 1995. Molecular cytogenetic analysis of the double-CMA3 chromosome of lake trout, Salvelinus namaycush. Cytogenet. Cell Genet. 70: 104–107.
Salvadori, S., A.M. Deiana, E. Coluccia, G. Floridia, E. Rossi & O. Zuffardi, 1995. Colocalization of (TTAGGG)n telomeric sequences and ribosomal genes in Atlantic eels. Chromosome Res. 3: 54–58.
Slijepcevic, P., Y. Xiao, I. Dominguez & A.T. Natarajan, 1996. Spontaneous and radiation-induced chromosomal breakage at interstitial telomeric sites. Chromosoma 104: 596–604.
Sola, L., A.R. Rossi, V. Iaselli, E.M. Rasch & P.J. Monaco, 1992. Cytogenetics of bisexual/unisexual species of Poecilia. II. Analysis of heterochromatin and nucleolar organizer regions in Poecilia mexicana mexicana by C-banding and DAPI, quinacrine, Chromomycin A3, and silver staining. Cytogenet. Cell Genet. 60: 229–235.
Sumner, A.T., 1972. A simple technique for demonstrating centromeric heterochromatin. Exp. Cell Res. 75: 304–306.
Takai, A. & Y. Ojima, 1986. Some features on nucleolus organizer regions in fish chromosomes, pp. 899–909 in Indo-Pacific Fish Biology: Proceedings of the Second International Conference of Indo-Pacific Fishes, edited by T. Uyeno, R. Arai, T. Taniuchi & K. Matsuura. Ichthyological Society of Japan, Tokyo.
Ueda, T., M. Hayashi, N. Koide, T. Sofuni & J. Kobayashi, 1991. Preliminary examination of the mutagenicity test using embryo cells of rose bitterling, Rhodeus ocellatus ocellatus. Chrom. Inf. Serv. 51: 12–14.
Ueda, T. & J. Kobayashi, 1991. Chromosomal polymorphisms in oily bitterling Acheilognathus limbatus. Chrom. Inf. Serv. 50: 13–14.
Ueda, T., N. Mashiko, H. Takizawa, Y. Akai, T. Ishinabe, R. Arai & H. Wu, 1997. Ag-NOR variation in chromosomes of Chinese bitterlings, Rhodeus lighti and Tanakia himantegus (Cypriniformes, Cyprinidae). Ichtyol. Res. 44: 302–305.
Ueda, T., H. Naoi & R. Arai, 2001. Flexibility on the karyotype evolution in bitterlings (Pisces, Cyprinidae). Genetica 111: 423–432.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sola, L., Gornung, E., Naoi, H. et al. FISH-Mapping of 18S Ribosomal RNA Genes and Telomeric Sequences in the Japanese Bitterlings Rhodeus ocellatus kurumeus and Tanakia limbata (Pisces, Cyprinidae) Reveals Significant Cytogenetic Differences in Morphologically Similar Karyotypes. Genetica 119, 99–106 (2003). https://doi.org/10.1023/A:1024446910161
Issue Date:
DOI: https://doi.org/10.1023/A:1024446910161