Abstract
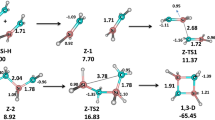
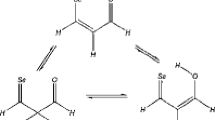
A new approach to determination of the stereochemical structure of bis-selenium-substituted alkenes using experimental 77Se NMR studies and B3LYP/6-311G(d) quantum-chemical calculations is developed. Joint analysis of experimental and calculated data allows assignment of signals in the 77Se NMR spectrum. The method was evaluated taking the model compounds (PhSe)HC=C(SePh)R (R = COOMe, CH2NMe2, CH2OH, Ph) as examples.
Similar content being viewed by others
References
Catalytic Heterofunctionalization, Eds. A. Togni and H. Grützmacher, Wiley-VCH, Weinheim, 2001.
I. P. Beletskaya and C. Moberg, Chem. Rev., 1999, 99, 3435.
O. N. Temkin, G. K. Shestakov, and Yu. A. Treger, Atsetilen: Khimiya. Mekhanizmy reaktsii. Tekhnologiya [Acetylene: Chemistry. Reaction Mechanisms. Technology], Ed. O. N. Temkin, Khimiya, Moscow, 1991, 416 pp. (in Russian).
A. Yamamoto, Organotransition Metal Chemistry, Wiley, New York, 1986.
Accurate Molecular Structure: Their Determination and Importance, Eds. A. Domenicano and I. Hargittai, Oxford University Press, Oxford, 1992.
D. Neuhaus and M. P. Williamson, The Nuclear Overhauser Effect in Structural and Conformational Analysis, VCH, New York, 1989.
M. P. Williamson, in Encyclopedia of Nuclear Magnetic Resonance, Eds. D. M. Grant and R. K. Harris, Wiley, Chichester, 1996, 5, p. 3262.
K. Wolinski, J. F. Hilton, and P. Pulay, J. Am. Chem. Soc., 1990, 112, 8251.
R. Ditchfield, Mol. Phys., 1974, 27, 789.
W. Koch and M. C. Holthausen, A Chemist's Guide to Density Functional Theory, Wiley-VCH, Weinheim, 2001, 300 pp.
T. Helgaker, M. Jaszunski, and K. Ruud, Chem. Rev., 1999, 99, 293.
J. R. Cheeseman, G. W. Trucks, T. A. Keith, and M. J. Frisch, J. Chem. Phys., 1996, 104, 5497.
G. Rauhut, S. Puyear, K. Wolinski, and P. Pulay, J. Phys. Chem., 1996, 100, 6310.
W. Nakanishi and S. Hayashi, J. Phys. Chem., A, 1999, 103, 6074.
V. P. Ananikov, I. P. Beletskaya, G. G. Aleksandrov, and I. L. Eremenko, Organometallics, 2003, 22, 1414.
H. Kuniyasu, A. Ogawa, S.-I. Miyazaki, I. Ryu, N. Kambe, and N. Sonoda, J. Am. Chem. Soc., 1991, 113, 9796.
G. Bodenhausen, H. Kogler, and R. R. Ernst J. Magn. Reson., 1984, 58, 370.
R. Wagner and S. Berger, J. Magn. Reson., Ser. A, 1996, 123, 119.
A. Bax and R. Freeman, J. Magn. Reson., 1981, 44, 542.
J. Ruiz-Cabello, G. W. Vuister, C. T. W. Moonen, P. van Gelderen, J. S. Cohen, and C. M. van Zijl, J. Magn. Reson., 1992, 100, 282.
W. Willker, D. Leibfritz, R. Kerssebaum, and W. Bermel, Magn. Reson. Chem., 1993, 31, 287.
A. D. Becke, Phys. Rev., A, 1988, 38, 3098.
C. Lee, W. Yang, and R. G. Parr, Phys. Rev., B, 1988, 37 , 785.
A. D. Becke, J. Chem. Phys., 1993, 98, 5648.
R. Ditchfield, W. J. Hehre, and J. A. Pople, J. Chem. Phys., 1971, 54, 724.
R. Krishnan, J. S. Binkley, R. Seeger, and J. A. Pople, J. Chem. Phys., 1980, 72, 650.
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, V. G. Zakrzewski, J. A. Montgomery Jr, R. E. Stratmann, J. C. Burant, S. Dapprich, J. M. Millam, A. D. Daniels, K. N. Kudin, M. C. Strain, O. Farkas, J. Tomasi, V. Barone, M. Cossi, R. Cammi, B. Mennucci, C. Pomelli, C. Adamo, S. Clifford, J. Ochterski, G. A. Petersson, P. Y. Ayala, Q. Cui, K. Morokuma, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. Cioslowski, J. V. Ortiz, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. Gomperts, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, C. Gonzalez, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, J. L. Andres, C. Gonzalez, M. Head-Gordon, E. S. Replogle, and J. A. Pople, GAUSSIAN-98, Rev. A.7, Gaussian, Pittsburgh (PA), 1998.
H. Duddeck, Prog. NMR Spectrosc., 1995, 27, 1.
H. Gunther, NMR Spectroscopy, John Wiley and Sons Ltd, Chichester, 1996, 581 pp.
M. H. Levitt, J. Magn. Reson., 1997, 126, 164.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ananikov, V.P., Beletskaya, I.P. New approach to stereochemical structure determination of bis-selenium-subsituted alkenes. Russian Chemical Bulletin 52, 811–816 (2003). https://doi.org/10.1023/A:1024423603695
Issue Date:
DOI: https://doi.org/10.1023/A:1024423603695