Abstract
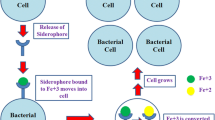
Different mono-, bis- or triscatecholates and mixed mono- or biscatecholate hydroxamates were synthesized as potential siderophores for mycobacteria. Siderophore activity was tested by growth promotion assays using wild type strains and iron transport mutants of mycobacteria as well as Gram-negative bacteria. Some triscatecholates and biscatecholate hydroxamates were active in mutants of Mycobacterium smegmatis deficient in mycobactin and exochelin biosynthesis or exochelin permease, respectively, indicating an uptake route independent of the exochelin/mycobactin pathway. Structure activity relationships were studied. Ampicillin conjugates of some of these compounds were inactive against mycobacteria but active against Gram-negative bacteria.
Similar content being viewed by others
References
Bergeron RJ, McGovern KA, Channing MA, Burton PS. 1980 Synthesis of N4-Acylated N1, N8-Bis(acyl)spermidines: An approach to the synthesis of siderophores. J Org Chem 45, 1589–1592.
Bergeron RJ, Kline SJ, Stollowich NJ, McGovern KA, Burton PS. 1981 Flexible synthesis of polyamine catecholamides. J Org Chem 46, 4524–4529.
Bird TGC, Arnould JC, Bertrandie A, Jung FH. 1992 Pharmacokinetics of catechol cephalosporins. The effect of incorporating substituents into the catechol moiety on pharmacokinetics in a marmoset model. J Med Chem 35, 2643–2651.
Drechsel H, Jung G. 1998 Peptide Siderophores. J Peptide Sci 4, 147–181.
Geffken D. 1986 Direkte O-Acylierung von Hydroxylamin und N-monosubstituierten Hydroxylaminen mit 1-Benzoylimidazol. Chem Ber 119, 744–746.
Heinisch L, Ulbricht H, Willitzer H, Hanschke KG, Tresselt D, MÖllmann U, Eckardt K, Haupt I. 1992 Synthesis and antibacterial activity of benzoylaminoacyl penicillins and related compounds with and without acylated catechol substituents. Arzneim-Forsch./Drug Res 42, 668–673.
Heinisch L, Gebhardt P, Heidersbach R, Reissbrodt R, MÖllmann U. 2002a New synthetic catecholate-type siderophores with triamine backbone. BioMetals 15, 133–144.
Heinisch L, Wittmann S, Stoiber T, Berg A, Ankel-Fuchs D, MÖllmann U. 2002b Highly antibacterial active aminoacylpenicillin conjugates with bis-catecholate siderophores based on secondary diamino acids and related compounds. J Med Chem 45, 3032–3040.
Heinisch L, Wittmann S, Stoiber T, Scherlitz-Hofmann I, Ankel-Fuchs D, MÖllmann U. 2002c New tris-and tetrakis-catecholate siderophores based on polyazaalkanoic acids and their ?-lactam conjugates. Arzneim-Forsch/Drug Res (in press).
Lin Y-M, Miller MJ, MÖllmann U. 2001 The remarkable hydrophobic effect of a fatty acid side chain on the microbial growth promoting activity of a synthetic siderophore. BioMetals 14, 153–157.
MuÑoz-Bellido J-L, Garcia Rodriguez JA. 1996 New trends in cephalosporin developments: Catechol and isoxazolidin cephalosporins. Infect Dis Clin Practise 5, 232–243.
Ratledge, C. 1999 Iron metabolism. In Ratledge C, and Dale J eds. Mycobacteria: molecular biology and virulence. 260–286. Oxford, United Kingdom: Blackwell Science Publishers Ltd.
Schnabelrauch M, Wittmann S, Rahn K, MÖllmann U, Reissbrodt R, Heinisch L. 2000 New synthetic catecholate-type siderophores based on amino acids and dipeptides. BioMetals 13, 333–348.
Schumann G, MÖllmann U, Heinemann I. 1998Mutants of Mycobacterium species and their use for screening of antibiotic vectors. Patent application DE 19817021.9 (17.4.1998) GrÜnenthal GmbH.
Schumann G, MÖllmann U. 2001 Screening system for xenosiderophores as potential drug delivery agents in mycobacteria. Antimicrob Ag Chemother 45, 1317–1322.
Schwyn B, Neilands JB. 1987 Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160, 47–56.
Sulsky R, Demers JP. Alkylation of N-benzyloxyurea and carbamates. 1989 Tetrahedron Lett 30, 31–34.
Wittmann S, Heinisch L, Scherlitz-Hofmann I, MÖllmann U, Ankel-Fuchs D. 2000 8-Acyloxy-1,3-benzoxazine-2,4-diones as siderophore components of antibiotics. Arzneim-Forsch/Drug Res 50, 752–757.
Wittmann S, Heinisch L, Scherlitz-Hofmann I, MÖllmann U. 2001 Neue Siderophoranaloga als 4-oder 6-zÄhnige Eisenchelatoren auf der Basis von AminosÄuren oder Peptiden, Verfahren zu ihrer Herstellung und ihre Anwendung. Ger Offen DE 10111161 A1.
Wittmann S, Schnabelrauch M, Scherlitz-Hofmann I, MÖllmann U, Ankel-Fuchs D, Heinisch L. 2002 New synthetic siderophores and their highly antibacterial active antibiotic conjugates based on diamino acids or dipeptides. Bioorg Med Chemistry 10, 1659–1670.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wittmann, S., Heinisch, L., Scherlitz-Hofmann, I. et al. Catecholates and mixed catecholate hydroxamates as artificial siderophores for mycobacteria. Biometals 17, 53–64 (2004). https://doi.org/10.1023/A:1024409517626
Issue Date:
DOI: https://doi.org/10.1023/A:1024409517626