Abstract
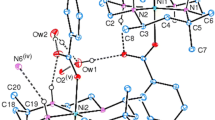
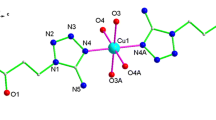
We report here two crystal structures of heavy metal complexes containing macrocyclic trithioethers. The trithioether, 10S3 (1,4,7-trithiacyclodecane) complexes Pb(II) to form a compound of the formula [Pb(10S3)](ClO4)2(H2O) (Compound 1). This behavior contrasts the related trithioether 9S3 (1,4,7-trithianonane), which readily forms a bis Pb(II) complex. Crystals of Compound 1 consist of [Pb(10S3)(H2O)]2+ units and perchlorate anions which are weakly bound to the Pb2+ center. The two perchlorate anions serve as bidentate ligands to a single lead(II) center. However, a third oxygen in one of the perchlorates also serves as a bridging group between two lead(II) centers, linking the [Pb(10S3)(H2O)]2+ units into zigzag chains. Thus, a highly irregular nine-coordinate (S3O6) environment around the Pb2+ion is formed by the three sulfur donors from the 10S3 ligand, one coordinated water, and five oxygens from three different perchlorate anions. Also, the lone pair electrons on the Pb center are stereochemically active. Compound 2, [Cd(9S3)2](ClO4)2 ⋅ 2 CH3NO2, forms a distorted octahedral structure with unusual hexakis(thioether) coordination to the Cd(II) center. Bond distances and angles are very comparable to those found in the structure of the tetrafluoroborate salt of the complex cation. (1): P21/c, a = 9.1720(6) Å, b = 15.7687(11) Å, c = 11.8427(8) Å, β = 93.0180(10) ○, V = 1710.4 (2) Å3, Z = 4; (2): P21/c , a = 10.4305(10) Å, b = 15.2059(6) Å, c = 9.4919(7) Å, β = 99.324(9)○, V = 1485.6(2) Å3, Z = 2.
Similar content being viewed by others
References
Blake, A.J.; Schröder, M. In Advances in Inorganic Chemistry; Sykes, A.G., Ed.; Academic Press: New York, 1990; Vol. 35, p 2.
Cooper, S.R. Acc. Chem. Res. 1988, 21, 141.
Cooper, S.; Rawle, S.C. Struct. Bonding (Berlin) 1990, 72, 1.
Schröder, M. Pure Appl. Chem. 1988, 60, 517.
Glass, R.S.; Steffen, L.K.; Swanson, D.D.; Wilson, G.S.; de Gelder, R.; de Graaff, R.A.G.; Reedijk, J. Inorg. Chim. Acta 1993, 207, 241.
Setzer, W.N.; Tang, Y.; Grant, G.J.; VanDerveer, D.G. Inorg. Chem. 1991, 30, 3652
Setzer, W.N.; Tang, Y.; Grant, G.J.; VanDerveer, D.G. Inorg. Chem. 1992, 31, 1116.
Helm, M.L.; Combs, C.M.; VanDerveer, D.G.; Grant, G.J. Inorg. Chim. Acta. 2002, 338C, 182.
Pickardt, J.; Shenn, J. Z. Naturforsch., Teil B 1993, 48, 969.
Küppers, H.-J.; Wieghardt, K.; Nuber, B.; Weiss, J. Z. Anorg. Allg. Chem. 1990, 577, 155.
Blake, A.J.; Fenske, D.; Li, W.-S.; Schröder, M. J. Chem. Soc., Dalton Trans. 1998, 3961.
Olmstead, M.M.; Kessler, R.M.; Hope, H.; Yanuck, M.D.; Musker, K.R. Acta Crystallogr., Sect. C. 1987, 43, 1890.
Setzer, W.N.; Cacioppo, E.L.; Guo, Q.; Grant, G.J.; Kim, D.D.; Hubbard, J.L.; VanDerveer, D.G. Inorg. Chem. 1990, 29, 2672.
Pregosin, P.S. In Transition Metal Nuclear Magnetic Resonance; Pregosin, P.S., Ed.; Elsevier: New York, 1991; p 293, and references cited therein.
Summers, M.F. Coord. Chem. Rev. 1988, 86, 43.
Harrison, P.G. In Encyclopedia of Inorganic Chemistry; King, R.B., (Ed.); Wiley: New York, 1997; Vol. 4, p 1944.
Setzer, W.N.; Guo, Q.; Grant, G.J.; Hubbard, J.L.; Glass, R.S.; VanDerveer, D.G. Heteroat. Chem. 1990, 4, 317.
SMART 5.054, SAINT 6.01, SHELXTL 5.1, Programs Used for Data Collection, Solution and Refinement of This Structure; Bruker AXS: Madison, WI, 1998-1999.
Blessing, R.H. Acta Crystallogr., Sect. A 1995, 51, 33.
SHELXS86, SHELX93, Programs Used for Data Collection, Solution and Refinement of This Structure; Bruker AXS: Madison, WI.
Hoffmann, P.; Hermes, F.-J.; Mattes, R. Z. Nautforsch., Teil B 1988, 43, 567.
Wieghardt, K.; Kleine-Boymann, M.; Nuber, B.; Weiss, J.; Zsolnai, L.; Huttner, G. Inorg. Chem. 1986, 251, 647
Huheey, J.E.; Keiter, E.A.; Keiter, R.L. Inorganic Chemistry: Principles of Structure and Reactivity, 4th ed.; Harper Collins: New York, 1993; p 116.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Helm, M.L., Loveday, K.D., Combs, C.M. et al. Heavy metal complexes of macrocyclic trithioethers. Journal of Chemical Crystallography 33, 447–455 (2003). https://doi.org/10.1023/A:1024294417454
Issue Date:
DOI: https://doi.org/10.1023/A:1024294417454