Abstract
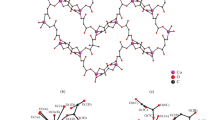
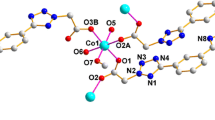
Reaction of the Zn, Cd, or Co nitrate salts with the deprotonated ligand (2-hydroxy-3-t-butyl-methylphenyl)bis(3,5-dimethylpyrazolyl)methane (L1O−) in methanol produced the following complexes: [(L1OH)Zn(NO3)2] in two isomorphs, a = 40.983(8) Å, b = 9.571(2) Å, c = 15.667(8) Å, α = 90○, β = 106.38(1)○, γ = 90○, C2/c, and a = 13.027(3) Å, b = 14.781(4) Å, c = 16.107(3) Å, α = 90○, β = 105.30(1)○, γ = 90○, P21/n; [(L1OH)Cd(pz)(NO3)2] a = 14.7476(2) Å, b = 13.5411(2) Å, c = 16.7223(2) Å, α = 90○, β = 110.3840(10)○, γ = 90○, P21/c; and [(L1O)Co(pz)(NO3)] a = 11.4240(2) Å, b = 13.4498(2) Å, c = 13.8056(2) Å, α = 105.2080(10)○, β = 105.8130(10)○, γ = 112.7470(10)○, P \({\bar 1}\). The Zn adopts a pseudotetrahedral four-coordinate geometry where the potentially tridentate ligand is actually bidentate with a protonated and uncoordinated phenoxy arm. The Co complex is pseudooctahedral six-coordinate where the phenoxy arm is deprotonated and coordinated. Finally the Cd complex is seven-coordinate but the metal is not coordinated through the phenoxy group that is again protonated.
Similar content being viewed by others
References
Lipscomb, W.N.; Straeter, N. Chem. Rev. 1996, 96, 2375.
Higgs, T.C.; Carrano, C.J. Inorg. Chem. 1997, 36, 291.
Han, R.; Parkin, G. J. Am. Chem. Soc. 1991, 113, 9707.
Kimblin, C.; Murphy, V.J.; Hascall, T.; Bridgewater, B.M.; Bonanno, J.B.; Parkin, G. Inorg. Chem., 2000, 39, 967.
Hammes, B.S.; Carrano, C.J. Inorg. Chem. 1999, 38, 4593.
Hammes, B.S.; Carrano, C.J. Inorg. Chim. Acta 2000, 300, 427.
Hammes, B.S.; Warthen, C.R.; Crans, D.C.; Carrano, C.J. J. Inorg. Biol Chem. 2000, 6, 82.
Park, H.I.; Ming, L. J. Biol. Inorg. Chem. [online]. 2002.
Hammes, B.S.; Luo, X.; Carrano, M.W.; Carrano, C.J. Manuscript submitted for publication.
Kime-Hunt, E.; Spartalian, K.; DeRusha, M.; Nunn, C.M.; Carrano, C.J. Inorg. Chem., 1989, 28, 4392.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shirin, Z., Hammes, B.S., Warthen, C.R. et al. A comparison of the coordination preferences of Zn, Co, and Cd(II) with the heteroscorpionate ligand (2-hydroxy-3-t-butyl-methylphenyl)bis (3,5-dimethylpyrazolyl)methane. Journal of Chemical Crystallography 33, 431–436 (2003). https://doi.org/10.1023/A:1024290316546
Issue Date:
DOI: https://doi.org/10.1023/A:1024290316546