Abstract
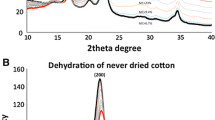
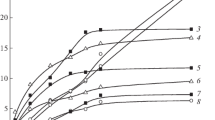
Structural changes in never- dried, disintegrated bacteria l cellulose by treatment with aqueous NaOH were examined by electron microscopy, X-ray diffractometry and acid hydrolysis behaviour and compared with those of cotton cellulose. The microfibril kept its fibrillar morphology after treatment with NaOH solutions of less than 9% (w/w), but changed into irregular aggregates when treated with NaOH above 12% (w/w), corresponding to the crystal conversion to cellulose II. The crystallinity of the resulting cellulose II was very low after a brief alkali treatment, but was increased significantly by elongated treatment (up to 10 days). In contrast, cotton cellulose was converted to cellulose II of fairly high crystallinity by alkali treatment of as little as 3 min duration, and the crystallinity did not change with longer treatments. The leveling-off degree of polymerization (LODP) of bacterial cellulose was decreased from 150 to 50 by 18% (w/w) NaOH treatment, while that of cotton linter decreased from 260 to 70. These characteristic differences between cotton linter cellulose and bacterial cellulose can be ascribed to a basic difference in microfibrillar organization in these materials: the microfibrils in cotton cellulose are in close contact with neighbouring microfibrils having opposite polarity, and in bacterial cellulose are isolated from each other and require chain folding to form the antiparallel cellulose II crystal
Similar content being viewed by others
REFERENCES
Alexander, W. J. and Mitchel, R. L. (1949) Rapid measurement of cellulose viscosity by nitration method. Anal. Chem. 21, 1497–1500.
Battista, O. A., Coppick, S., Howpman, J. A., Morehead, F. F. and Sisson, W. A. (1956) Level-off degree of polymerization, relation to polyphase structure of cellulose fibers. Ind. Eng. Chem. 48, 333–335.
Brown, R. M. Jr, Haigler, C. H., Suttie, J., White, A. R., Roberts, E., Smith, C., Itoh, T. and Cooper, K. (1983) The biogenesis and degradation of cellulose. J. Appl. Polym. Sci. Appl. Polym. Symp. 37, 33–78.
Chanzy, H. D. and Roche, E. J. (1975) Fibrous mercerization of Valoniacellulose. J. Polym. Sci. Polym. Phys. Ed. 13, 1859–1862.
Chanzy, H. and Henrissat, B. (1985) Undirectional degradation of Valoniacellulose microcrystals subjected to cellulase action. FEBS Lett. 184, 285–288.
Dolmetsh, H. (1970) Regular physical changes of the fibrillar structure in spun fibers of cellulose and synthetic polymers. Melliand textilber 51, 182–190.
Gessler, K., Krauss, N., Steiner, T., Betzel, C., Sandmann, C. and Saenger, W. (1994) Crystal structure of •-D-cellotetraose hemihydrate with implications for the structure of cellulose II. Science 266, 1027–1029.
Haigler, C. H. (1985) The functions and biogenesis of native cellulose. In Cellulose Chemistry and Its Applications (T. P. Nevell and S. H. Zeronian eds) Chichester, UK: Ellis Horwood, pp. 30–83.
Hayashi, J., Nakagawa, J. and Asano, S. (1987) (110) molecular sheet structure of cellulose. Proceedings of 1987 International Dissolving Pulps Conference, March 1987, Geneva, Switzerland, 193–200.
Hayashi, J., Yamada, T., Kimura, K. (1976) The change of the chain conformation of cellulose from type I to II. Appl. Polym. Symp. 28, 713–727.
Hieta, K., Kuga, S. and Usuda, M. (1984) Electron staining of reducing ends evidences a parallel-chain structure in Valoniacellulose. Biopolymers 23, 1807–1810.
Hutino, K. and Sakurada, I. (1940) A fourth modification of cellulose. Naturwiss. 28, 577–578.
Immergut, E. A. and Ranby, B. G. (1956) Heterogeneous acid hydrolysis of native cellulose fibers. Ind. Eng. Chem. 48(7), 1183–1189.
Immergut, E. H., Ranby, B. G. and Mark, H. F. (1953) Recent work on molecular weight of cellulose. Ind. Eng. Chem. 45(11), 2483–2490.
Jeffries, R. and Warwicker, J. O. (1969) The function of swelling in the finishing of cotton. Textile Res. J. 39, 548–559.
Kerr, A. J. and Goring, D. A. I. (1975) The ultrastructural arrangement of the wood cell wall. Cellulose Chem. Technol. 9, 563–573.
Kuga, S. and Brown, R. M. Jr. (1988) Silver labeling of the reducing ends of bacterial cellulose. Carbohydr. Res. 180, 345–350.
Kuga, S., Takagi, S. and Brown, R. M. Jr. (1993) Native folded-chain cellulose II. Polymer 34, (15) 3293–3297.
Legrand, C. (1951) Cellulose III regenerated from ammonia-cellulose. J. Polym. Sci. 7, 333–339.
Loeb, L. and Segal, L. (1954) Preparation of cotton cellulose (IV) from cotton cellulose (III). J. Polym. Sci. 14, 121–123.
Meyerhoff, G. and Jovanovic, S. (1967) Gel permeation chromatography, calibration, and distributions of cellulose trinitrates. Polym. Lett. 5, 495–499.
Morehead, F. F. (1950) Ultrasonic distintegration of cellulose fibers before and after hydrolysis. Textile Res. J. 20, 549–553.
Nishikawa, S. and Ono, S. (1913) Proc. Tokyo Mathematico-Physical Soc., 2nd series 7, 131.
Okano, T. and Sarko, A. (1985) Mercerization of cellulose. II. Alkali-cellulose intermediates and a possible mercerization mechanism. J. Appl. Polym. Sci. 30, 325–332.
Ranby, B. G. (1952) The cellular micelles. Tappi 35, 53–58.
Raymond, S., Heyraud, A., Tran Qui, Kvick, A. and Chanzy, H. (1995a) Crystal and molecular structure of •-D-cellotetraose hemihydrate as a model of cellulose II. Macromolecules 28, 2096–2100.
Raymond, S., Kvick, A. and Chanzy, H. (1995b) The structure of cellulose II: A revisit. Macromolecules 28, 8422–8425.
Sarko, A. (1976) Crystalline polymorphs of cellulose: prediction of structure and properties. Appl. Polym. Symp. 28, 729–742.
Sarko, A. (1978) What is the crystalline structure of cellulose? Tappi 61, 59–61.
Schramek, W., Neumann, H. and Schubert, C. (1933) The X-ray fiber diagram as a quantitative measure of changes in the micelles of cellulose by chemical process. Z. Physik. Chem. B. 20, 209–216.
Schramm, M. and Hestrin, S. (1954) Synthesis of cellulose by Acetobacter xylinum. Biochemistry, 58, 345–352.
Segal, L., Loeb, L. and Creely, J. (1954) X-ray studies of the decomposition product of the ethylamine-cellulose complex. J. Polym. Sci. 13, 193–206.
Sharples, A. (1957) The hydrolysis of cellulose and its relation to structure. Trans. Faraday Soc. 53, 1003–1013.
Sharples, A. (1958) Hydrolysis of cellulose and its relation to structure. II. Trans. Faraday Soc. 54, 913–917.
Shibazaki, H., Kuga, S., Onabe, F. and Brown, R. M. Jr. (1995) Acid hydrolysis behaviour of microbial cellulose II. Polymer 36(26), 4971–4976.
Sobue, H., Kiessig, H. and Hess, K. (1939) The system: cellulose-sodium hydroxide-water in relation to the temperature. Z Physik. Chem. B. 43, 309–328.
Sugiyama, J., Harada, H., Fujiyoshi, Y. and Uyeda, N. (1985) Lattice images from ultrathin sections of cellulose microfibrils in the cell wall of Valonia macrophysa Ku¨tz. P lanta 166, 161–168.
Sugiyama, J., Vuong, R. and Chanzy, H. (1991) Electron diffraction study on the two crystalline phases occurring in native cellulose from an algal cell wall. Macromolecules 24, 4168–4175.
Trogus, C. and Hess, K. (1935) X-ray studies of cellotriose and its derivatives. Ber. 68B, 1605–1610.
Warwicker, J. O. and Wright, A. C. (1967) Function of sheets of cellulose chains in swelling reactions on cellulose. J. Appl. Polym. Sci. 11, 659–671.
Yachi, T., Hayashi, J., Takai, M., Shimizu, Y. (1983) Supermolecular structure of cellulose: stepwise decrease in LODP and particle size of cellulose hydrolyzed after chemical treatment. J. Appl. Polym. Sci. Appl. Polym. Symp. 37, 325–343.
Yokota, H., Sei, T., Horii, F. and Kitamaru, R. (1990) 13C CP/MAS NMR study on alkali cellulose,J. Appl. Polym. Sci. 41, 783–791.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shibazaki, H., Kuga, S. & Okano, T. Mercerization and acid hydrolysis of bacterial cellulose. Cellulose 4, 75–87 (1997). https://doi.org/10.1023/A:1024273218783
Issue Date:
DOI: https://doi.org/10.1023/A:1024273218783