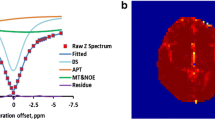
Abstract
A comparison between data from proton-MR spectroscopy (1HMRS) and quantitative histomorphology of tumor cell nuclei in gliomas has not been reported up to now. Therefore, the question must be answered, if there are any significant correlations between histomorphology of gliomas and quantitative data from 1HMRS concerning tissue metabolites.
Surgical glioma specimen (glioblastomas, astrocytomas, oligodendrogliomas) from 46 patients with tumor grades II–IV according to WHO have been evaluated by means of a digital image analysis system using Ki-67-immunostained paraffin sections. Nuclear density, Ki-67-proliferation index, nuclear area and shape variables (roundness factor, Fourier-amplitudes) have been determined from 200 randomly selected tumor cell nuclei in each tumor specimen. These data have been correlated with preoperative data from 1HMRS.
A positive correlation between Fourier-amplitudes, choline peak and lipide peak was observed, as well as a negative correlation between these variables and the nuclear roundness factor. This result indicates higher choline and lipide peaks with increasing irregularity of nuclear outlines. Proliferation index Ki-67 was positively correlated with the lipide peak, nuclear density showed a positive correlation with the choline peak. Glioblastomas (n = 29) showed an additional positive correlation between mean nuclear size and total creatine. Anaplastic gliomas (n = 12) showed a positive correlation between lactate peak and the standard deviation of the nuclear roundness factor. Further multivariate analyses have shown, that for the present collective of 46 cases, histometric variables have a higher significance than spectroscopic data for the differentiation of the different tumor grades.
These results verify a significant correlation between preoperative data from 1HMRS and histomorphology of tumor cell nuclei in gliomas, supporting the biological significance of both histomorphometry and 1HMRS for the evaluation of these tumors.
Similar content being viewed by others
References
Graves EE, Nelson SJ, Vigneron DB, Chin C, Verhey L, McDermott M, Larson D, Sneed PK, Chang S, Prados MD, Lamborn K, Dillon WP: A preliminary study of the prognostic value of proton magnetic resonance spectroscopic imaging in gamma knife radiosurgery of recurrent malignant gliomas. Neurosurgery 46: 319–328, 2000
Gupta RK, Cloughesy TF, Sinha U, Garakian J, Lazareff J, Rubino G, Rubino L, Becker DP, Vinters HV, Alger JR: Relationships between choline magnetic resonance spectroscopy, apparent diffusion coefficient and quantitative histopathology in human glioma. J Neuro-Oncol 50: 215–226, 2000
Norfray JF, Tomita T, Byrd SE, Ross BD, Berger PA, Miller RS: Clinical impact of MR spectroscopy when MR imaging is indeterminate for pediatric brain tumors. Am J Radiol 173: 119–125, 1999
Kleihues P, Cavenee WK: Pathology & Genetics - Tumors of the Nervous System. IARC Press, Lyon, 2000, pp 27–39
Bottomley PA: Spatial localization in NMR spectroscopy in vivo. Ann NY Acad Sci 508: 333–348, 1987
Haase A, Frahm J, H¨anicke W, Matthei D: 1H NMR chemical shift selective (CHESS) imaging. Phys Med Biol 30: 341–344, 1985
MRUI Home Page. See: http://www.mrui.uab.es/mrui/ mruiHomePage.html
De Beer, Van den Boogaart A, van Ormondt D, Pijnappel WW, den Hollander JA, Marien AJ: Application of time domain fitting in the quantification of in vivo 1H spectroscopic imaging data sets. NMR Biomed 5: 171–178, 1992
Van der Veen JWC, De Beer PR, Luyten PR, Van Ormondt D: Accurate quantification of in vivo 31P-NMR signals using the variable projection method and prior knowledge. Magn Reson Med 6: 92–98, 1988
Petrow OAC, Spencer DD, Alger JR, Prichard JW: High field proton magnetic resonance spectroscopy of human cerebrum obtained during surgery for epilepsy. Neurology 39: 1197–1202, 1989
Klunk WE, Xu CJ, Panchalingham K, Mc Clure RJ, Pettegrew JW: Analysis of magnetic resonance spectra by mole percent: comparison of absolute units. Neurobiol Aging 15: 133–140, 1994
Magnetom Vision Application-Manual, Numaris Version 31C. Siemens AG, Erlangen, Germany, 1999, pp 3–40
Nafe R, Schlote W, Schneider B: Planimetric characterization of tumor cell nuclei in astrocytic tumors - comparison of different tumor grades according to the WHO-classification. Electron J Pathol 6, 004–01.txt, 2000
Nafe R, Glienke W, Schlote W, Schneider B: EGFR geneamplification in glioblastomas - is there a relation with morphology of tumor cell nuclei and proliferative activity? Anal Quant Cytol Histol 23: 135–143, 2001
Nafe R, Schlote W, Schneider B: Data analysis for comparative histometry in pathology - I. Classification procedures and other statistical methods. Electron J Pathol 6, 002–03.txt, 2000
Hagberg G, Burlina AP, Mader I, Roser W, Radue EW, Seelig J: In vivo protonMRspectroscopy of human gliomas: definition of metabolic coordinates for multidimensional classification. MRM 34: 242–252, 1995
Lin A, Bluml S, Mamelak AN: Efficacy of proton magnetic resonance spectroscopy in clinical decision making for patients with suspected malignant brain tumors. J Neuro-Oncol 45: 69–81, 1999
McBride DQ, Miller BL, Nikas DL, Buchthal S, Chang L, Chiang F, Booth RA: Analysis of brain tumors using 1H magnetic resonance spectroscopy. Surg Neurol 44: 137–144, 1995
Usenius JP, Vainio P, Hernesniemi J, Kauppinen RA: Choline-containing compounds in human astrocytomas studied by 1H-NMR spectroscopy in vivo and in vitro. J Neurochem 63: 1538–1543, 1994
Byrd SE, Tomita T, Palka PS, Darling CF, Norfray JP, Fan J: Magnetic resonance spectroscopy (MRS) in the evaluation of pediatric brain tumors, part II: clinical analysis. J Natl Med Assoc 88: 717–723, 1996
Falini A, Calabrese G, Origgi D, Lipari S, Triulzi F, Losa M, Scotti G: Proton magnetic resonance spectroscopy and intracranial tumours: clinical perspectives. J Neurol 243: 706–714, 1996
Meyerand ME, Pipas JM, Mamourian A, Tosteson TD, Dunn JF: Classification of biopsy-confirmed brain tumors using single-voxel MR spectroscopy. Am J Neuroradiol 20: 117–123, 1999
Negendank WG, Sauter R, Brown TR, Evelhoch JL, Falini A, Gotsis ED, Heerschap A, Kamada K, Lee BCP, Mengeot MM, Moser E, Padavic-Shaller KA, Sanders JA, Spraggins TA, Stillman AE, Terwey B, Vogl TJ, Wicklow K, Zimmerman RA: Proton magnetic resonance spectroscopy in patients with glial tumors: a multicenter study. J Neurosurg 84: 449–458, 1996
Shimizu H, Kumabe T, Tominaga T, Kayama T, Hara K, Ono Y, Sato K, Arai N, Fujiwara S, Yoshimoto T: Noninvasive evaluation of malignancy of brain tumors with proton MR spectroscopy. Am J Neuroradiol 17: 737–747, 1996
Castillo M, Smith JK, Kwock L: Correlation of myoinositol levels and grading of cerebral astrocytomas. Am J Neuroradiol 21: 1645–1649, 2000
Carpinelli G, Carapella CM, Palombi L, Raus L, Caroli F, Podo F: Differentiation of glioblastoma multiforme from astrocytomas by in vitro 1H MRS analysis of human brain tumors. Anticancer Res 16: 1559–1564, 1996
Carapella CM, Carpinelli G, Knijn A, Raus L, Caroli F, Podo F: Potential role of in vitro 1H-magnetic resonance spectroscopy in the definition of malignancy grading of human neuroepithelial brain tumours. Acta Neurochir (Suppl) 68: 127–132, 1997
Go KG, Kamman RL, Pruim J, Hew JM, Vaalburg W, Paans AMJ, Mooyaart EL, Heesters MAAM, Lopes da Silva FH: On the principles underlying the diagnosis of brain tumours - A survey article. Acta Neurochir (Wien) 135: 1–11, 1995
Bendszus M, Warmuth-Metz M, Klein R, Burger R, Schichor C, Tonn JC, Solymosi L: MR spectroscopy in gliomatosis cerebri. Am J Neuroradiol 21: 375–380, 2000
Girard N, Wang ZJ, Erbetta A, Sutton LN, Phillips PC, Rorke LB, Zimmerman RA: Prognostic value of proton MRspectroscopy of cerebral hemisphere tumors in children. Neuroradiology 40: 121–125, 1998
Lazareff JA, Bockhorst KHJ, Curran J, Olmstead C, Alger JR: Pediatric low-grade gliomas: prognosis with proton magnetic resonance spectroscopic imaging. Neurosurgery 43: 809–818, 1998
Lazareff JA, Gupta RK, Alger JR: Variation of posttreatment H-MRSI choline signal intensity in pediatric gliomas. J Neuro-Oncol 41: 291–298, 1999
Norfray JF, Darling C, Byrd S, Ross BD, Schwalm C, Miller R, Tomita T: Short TE proton MRS and neurofibromatosis type 1 intracranial lesions. J Comput Assist Tomogr 23: 994–1003, 1999
Tedeschi G, Lundbom N, Raman R, Bonavita S, Duyn JH, Alger JR, diChiro G: Increased choline signal coinciding with malignant degeneration of cerebral gliomas: a serial proton magnetic resonance spectroscopy imaging study. J Neurosurg 87: 516–524, 1997
Tamiya T, Kinoshita K, Ono Y, Matsumoto K, Furuta T, Ohmoto T: Proton magnetic resonance spectroscopy reflects cellular proliferative activity in astrocytomas. Neuroradiology 42: 333–338, 2000
Parivar F, Hricak H, Shinohara K, Kurhanewicz J, Vigneron DB, Nelson SJ, Carroll PR: Detection of locally recurrent prostate cancer after cryosurgery: evaluation by transrectal ultrasound, magnetic resonance imaging and three-dimensional proton magnetic resonance spectroscopy. Urology 48: 594–599, 1996
Wefer AE, Hricak H, Vigneron DB, Coakley FV, Lu Y, Wefer J, Mueller-Lisse U, Carroll PR, Kurhanewicz J: Sextant localization of prostate cancer: comparison of sextant biopsy, magnetic resonance imaging and magnetic resonance spectroscopic imaging with step section histology. J Urol 164: 400–404, 2000
Cheng LL, Ma MJ, Becerra L, Ptak T, Tracey I, Lackner A, Gonz´alez RG: Quantitative neuropathology by high-resolution magic angle spinning proton magnetic resonance spectroscopy. Proc Natl Acad Sci USA 94: 6408–6413, 1997
Woermann FG, McLean MA, Bartlett PA, Parker GJ, Barker GJ, Duncan JS: Short echo time single-voxel 1H magnetic resonance spectroscopy in magnetic resonance imaging - negative temporal lobe epilepsy: different biochemical profile compared with hippocampal sclerosis. Ann Neurol 45: 369–376, 1999
Gerdes J, Lemke H, B aisch H, Wacker HH, Schwab U, Stein H: Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol 133: 1710–1715, 1984.
Shimizu H, Kumabe T, Shirane R, Yoshimoto T: Correlation between choline level measured by protonMRspectroscopy and Ki-67 labelling index in gliomas. Am J Neuroradiol 21: 659–665, 2000
Kuesel AC, Sutherland GR, Halliday W, Smith IC: 1H-MRS of high grade astrocytomas: mobile lipid accumulation in necrotic tissue. NMR Biomed 7: 149–155, 1994
Nafe R, Schlote W, Schneider B: Shape analysis of tumor cell nuclei in ependymomas by means of Fourier-analysis. Anal Quant Cytol Histol 22: 475–482, 2000
Bhakoo KK, Williams SR, Florian CL, Land H, Noble MD: Immortalization and transformation are associated with specific alterations in choline metabolism. Cancer Res 56: 4630–4635, 1996
Chang L, McBride D, Miller BL, Cornford M, Booth RA, Buchthal SD, Ernst TM, Jenden DJ: Localized in vivo 1H magnetic resonance spectroscopy and in vitro analyses of heterogeneous brain tumors. J Neuroimaging 5: 157–163, 1995
Miller BL: A review of chemical issues in 1H-NMR spectroscopy: N-acetyl-L-aspartate, creatine and choline. NMR Biomed 4: 47–52, 1991
Miller BL, Chang L, Booth RA, Ernst T, Cornford M, Nikas D, McBride D, Jenden DJ: In vivo 1H MRS choline: correlation with in vitro chemistry/histology. Life Sci 58: 1929-1935, 1996
Aiken NR, Gillies RJ: Phosphomonoester metabolism as a function of cell proliferative status and exogeneous precursors. Anticancer Res 16: 1393–1397, 1996
Demaerel P, Johannik K, vanHecke P, vanOngeval C, Verellen S, Marchal G, Wilms G, Plets C, Goffin J, van Calenbergh F, Lammens M, Baert AL: Localized 1H-NMR spectroscopy in fifty cases of newly diagnosed intracranial tumors. J Comput Assist Tomogr 15: 67–76, 1991
Luyten PR, Marien AJ, Heindel W, vanGerwen PH, Herholz K, denHollander JA, Friedmann G, Heiss WD: Metabolic imaging of patients with intracranial tumors: 1H-MR spectroscopic imaging and PET. Radiology 176: 791–799, 1990
Miller BL, McBride D, Riedy G, Caron M, Lipcamon J, O'Brien D: Changes in brain choline in 1H-MR spectroscopy. Bull Clin Neurosci 55: 115–122, 1990
Alger JR, Frank JA, Bizzi A, Fulham MJ, DeSouza BX, Duhaney MO, Inscoe SW, Black JL, vanZijl PCM, Moonen CTW, DiChiro G: Metabolism of human gliomas: assessment with 1H-MR spectroscopy and F-18-Fluorodeoxyglucose PET. Radiology 177: 633–641, 1990
McCoy CL, McIntyre DJO, Robinson SP, Aboagye EO, Griffith JR: Magnetic resonance spectroscopy and imaging methods for measuring tumor and tissue oxygenation. Br J Cancer (Suppl) 74: 226–231, 1996
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nafe, R., Herminghaus, S., Raab, P. et al. Preoperative Proton-MR Spectroscopy of Gliomas – Correlation with Quantitative Nuclear Morphology in Surgical Specimen. J Neurooncol 63, 233–245 (2003). https://doi.org/10.1023/A:1024249232454
Issue Date:
DOI: https://doi.org/10.1023/A:1024249232454