Abstract
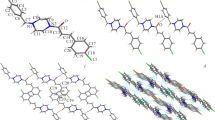
The crystal structures of threo-ifenprodil [threo-2-(4-benzyl-piperidin-1-yl)-1-(4-hydroxyphenyl)-propan-1-ol] (1), potent NMDA receptor antagonist, and two related, biologically active compounds: threo-2-(4-benzyl-piperidin-1-yl)-1-(4-fluorophenyl)-propan-1-ol (2) and 4-benzyl-1-phenethyl-piperidinium chloride (3) have been determined by X-ray structure analysis of single crystals. Compound 1 crystallizes in an orthorhombic system, with a space group Pna21 (a = 34.843(3) Å, b = 6.0261(13) Å, c = 8.9343(9) Å), compound 2 – in a monoclinic space group P21/c (a = 14.194(1) Å, b = 6.2831(6) Å, c = 20.948(1) Å, β = 101.762(6)○), and compound 3 – in an orthorhombic space group Pbca (a = 10.4627(8) Å, b = 11.2166(6) Å, c = 31.669(2) Å). The piperidine rings are close to ideal chair conformations, the substituents are in equatorial positions. Overall shapes of the molecules, defined by the dihedral angles between the terminal aromatic rings, are very similar in all three cases. In 1 and 3 the crystal packing is mainly determined by the hydrogen bonds, while in case of 2 – by weak van der Waals interactions. The molecules of 1 and 3 are disordered, and the disorder is caused by a rotation around the N–C bond.
Similar content being viewed by others
References
Carter, C.; Benavides, J.; Legendre, P.; Vincent, J.D.; Noel, F.; Thuret, F.; Lloyd, K.G.; Arbilla, S.; Zivkovic, B.; MacKenzie, E.T.; Scatton, B.; Langer, S.Z. J. Pharmacol. Exp. Ther. 1988, 247, 1222.
Chenard, B.L.; Shalaby, I.A.; Koe, B.K.; Ronau, R.T.; Butler, T.W.; Prochniak, M.A.; Schmidt, A.W.; Fox, C.B. J. Med. Chem. 1991, 34, 3085.
Carter, C.W.; Rivy, J.-P.; Scatton, B. Eur. J. Pharmacol. 1989, 164, 611.
Reynolds, I.J.; Miller, R.J. Mol. Pharmacol. 1989, 36, 758.
Williams, K. Mol. Pharmacol. 1993, 44, 851.
Whittmore, E.R.; Ilyin, V.I.; Woodward, R.M. J. Pharmacol. Exp. Ther. 1997, 282, 326.
CAD-4 Software, version 5; Enraf-Nonius: Delft, The Netherlands, 1989.
Rettig, S. ENPROC, Data Reduction Program for Enraf-Nonus CAD-4F Diffractometer; University of British Columbia: Vancouver, BC, Canada, 1978.
Sheldrick, G.M. Acta Crystallogr. 1990, A46, 467.
Sheldrick, G.M. SHELXL93, Program for the Refinement of Crystal Structures; University of Göttingen: Germany, 1993.
Stereochemical Workstation; Siemens Analytical X-ray Instruments Inc.: Madison, WI, 1989.
Duax, W.L.; Norton, D.A. Atlas of Steroid Structure, Vol. 1; Plenum: New York, 1975.
Allen, F.H.; Kennard, O.; Watson, D.G.; Brammer, L.; Orpen, A.G.; Taylor, R. In International Tables for Crystallography, Vol. C: Mathematical, Physical and Chemical Tables; Wilson, A.J.C., ed.; Kluwer: Dordrecht, The Netherlands, 1995; pp 685-706.
Domenicano, A.; Murray-Rust, P. Tetrahedron Lett., 1979, 24, 2283.
Steiner, T. Acta Crystallogr. 1998, B54, 456.
Desiraju, G.R.; Steiner, T. The Weak Hydrogen Bond; Oxford University Press: Oxford, 1999; pp 246-253.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kubicki, M., Codding, P.W. The crystal and molecular structures of rac-threo-ifenprodil and two other 4-benzylpiperidinyl derivatives. Journal of Chemical Crystallography 33, 563–568 (2003). https://doi.org/10.1023/A:1024242820066
Issue Date:
DOI: https://doi.org/10.1023/A:1024242820066