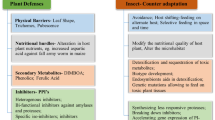
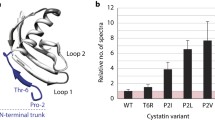
Abstract
Protease inhibitors expressed in transgenic plants can provide enhanced levels of resistance to important pest species. A sequential approach for testing the effects of protease inhibitor-expressing crops on nontarget herbivorous insects has been developed. The approach consists of five tiers. The first two tiers comprise the selection phase. In tier one, field surveys are used to characterise the nontarget invertebrate fauna of a crop. In tier 2, histochemical assays are used to identify the subset of herbivores with a particular class of digestive proteolytic enzymes. In the assessment phase a combination of laboratory ‘worst-case scenario’ studies (tier 3) and controlled environment or small-scale field trials (tier 4) are used to evaluate the impact of the protease inhibitor-expressing plants on the selected nontarget species. In the final tier, field trials are used to compare the relative effect of transgenic plants and current management practices, such as pesticide use, on selected species. The first four tiers of the approach are described using potatoes expressing cystatins, a family of cysteine proteinase inhibitors, as an example. Although the plants have enhanced levels of resistance to potato cyst nematodes (PCN), Globodera pallida and Globodera rostochiensis, the results establish that they have negligible impact on the nontarget herbivorous insect, Eupteryx aurata.
Similar content being viewed by others
References
Ashouri A, Overney S, Michaud D and Cloutier C (1998) Fitness and feeding are affected in the two-spotted stinkbug, Perillus bioculatus, by the cysteine proteinase inhibitor, oryzacystatin I. Archives of Insect Biochemistry and Physiology 38(2): 74–83.
Barrett KL, Grandy N, Harrison NG, Hassan SA and Oomen PA (1994) Guidance document on regulatory testing procedures for pesticides and non-target arthropods. In: Proceedings of the ESCORT Workshop. Society of Environmental Toxicology and Chemistry, IAC Wageningen, The Netherlands.
Birch ANE, Geoghegan IE, Majerus MEN, McNicol JW, Hackett CA, Gatehouse AMR and Gatehouse JA (1999) Tritrophic interactions involving pest aphids, predatory 2-spot ladybirds and transgenic potatoes expressing snowdrop lectin for aphid resistance. Mol Breed 5(1): 75–83.
Christeller JT, Burgess EPJ, Mett V, Gatehouse HS, Markwick NP, Murray C, Malone LA, Wright MA, Philip BA, Watt D, Gatehouse LN, Lovei GL, Shannon AL, Phung MM, Watson LM and Laing WA (2002) The expression of a mammalian proteinase inhibitor, bovine spleen trypsin inhibitor in tobacco and its effects on Helicoverpa armigera larvae. Transgenic Res 11(2): 161–173.
Couty A, Down RE, Gatehouse AMR, Kaiser L, Pham-Delegue MH and Poppy GM (2001) Effects of artificial diet containing GNA and GNA-expressing potatoes on the development of the aphid parasitoid Aphidius ervi Haliday (Hymenoptera: Aphidiidae). J Insect Physiol 47(12): 1357–1366.
Cowgill SE, Wright C and Atkinson HJ (2002a) Transgenic potatoes with enhanced levels of nematode resistance do not have altered susceptibility to nontarget aphids. Mol Ecol 11: 821–827.
Cowgill SE, Bardgett RD, Kiezebrink DT and Atkinson HJ (2002b) The effect of transgenic nematode resistance on nontarget organisms in the potato rhizosphere. J Appl Ecol 39: 915–923.
Dohmen GP (1998) Comparing pesticide effects on beneficials in a sequential testing scheme. In: McEwen P (ed.), Ecotoxicology: Pesticides and Beneficial Organisms. (pp.92–109) Kluwer Academic Publishers, Dordrecht.
Donegan KK, S eidler RJ, Fieland VJ, Schaller DL, Palm CJ, Ganio LM, Cardwell DM and Steinberger Y (1997) Decomposition of genetically engineered tobacco under field conditions: persistence of the proteinase inhibitor I product and effects on soil microbial respiration and protozoa, nematode and microarthropod populations.J Appl Ecol 34(3): 767–777.
Down RE, Ford L, Woodhouse SD, Raemaekers RJM, Leitch B, Gatehouse JA and Gatehouse AMR (2000) Snowdrop lectin (GNA) has no acute toxic effects on a beneficial insect predator, the 2-spot ladybird (Adalia bipunctata L.). J Insect Physiol 46(4): 379–391.
Girard C, Bonade-Bottino CM, Pham-Delegue MH and Jouanin L (1998a) Two strains of cabbage seed weevil (Coleoptera: Curculionidae) exhibit differential susceptibility to a transgenic oilseed rape expressing oryzacystatin I. J Insect Physiol 44(7-8): 569–577.
Girard C, PicardNizou AL, Grallien E, Zaccomer B, Jouanin L and PhamDelegue MH (1998b) Effects of proteinase inhibitor ingestion on survival, learning abilities and digestive proteinases of the honeybee. Transgenic Res 7(4): 239–246.
Griffiths BS, Geoghegan IE and Robertson WM (2000) Testing genetically engineered potato, producing the lectins GNA and Con A, on non-target soil organisms and processes. J Appl Ecol 37(1): 159–170.
Head G, Brown CR, Groth ME and Duan JJ (2001) Cry1Ab protein levels in phytophagous insects feeding on transgenic corn: implications for secondary exposure risk assessment. Entomol Exp Appl 99(1): 37–45.
Hellmich RL, Siegfried BD, Sears MK, Stanley-Horn DE, Daniels MJ, Mattila HR, Spencer T, Bidne KG and Lewis LC (2001) Monarch larvae sensitivity to Bacillus thuringiensis-purified proteins and pollen. Proc Natl Acad Sci USA 98(21): 11925–11930.
Herms CP, McCullough DG, Ba uer LS, Haack RA, Miller DL and Dubois NR (1997) Susceptibility of the endangered Karner blue butterfly (Lepidoptera: Lycaenidae) to Bacillus thuringiensis var kurstaki used for gypsy moth suppression in Michigan. Great Lakes Entomol 30(4): 125–141.
Hilbeck A, Moar WJ, Pusztai-Carey M, Filippini A and Bigler F (1998) Toxicity of Bacillus thuringiensis Cry1Ab toxin to the predator Chrysoperla carnea (Neuroptera: Chrysopidae). Environ Entomol 27(5): 1255–1263.
Hilder VA, G atehouse AMR, Sheerman SE, Barker RF and Boulter D (1987) A Novel Mechanism of insect resistance engineered into tobacco. Nature 330(6144): 160–163.
Holland JM and Thomas SR (1997) Quantifying the impact of polyphagous invertebrate predators in controlling cereal aphids and in preventing wheat yield and quality reductions. Ann Appl Biol 131(3): 375–397.
Jepson PC, Croft BC and Pratt GE (1994) Test systems to determine the ecological risks posed by toxin release from Bacillus thuringiensis genes in crop plants. Mol Ecol 3(1): 81–89.
Johnson R, Narvaez J, An GH and Ryan C (1989) Expression of proteinase inhibitor-I and inhibitor-II in transgenic tobacco plants - effects on natural defense against Manduca sexta larvae. Proc Natl Acad Sci USA 86(24): 9871–9875.
Lee SI, Lee SH, Koo JC, Chun HJ, Lim CO, Mun JH, Song YH and Cho MJ (1999) Soybean Kunitz trypsin inhibitor (SKTI) confers resistance to the brown planthopper (Nilaparvata lugens Stal) in transgenic rice. Mol Breed 5(1): 1–9.
Leple JC, Bonadebottino M, Augustin S, Pilate G, Letan VD, Delplanque A, Cornu D and Jouanin L (1995) Toxicity to Chrysomela tremulae (Coleoptera: Chrysomelidae) of transgenic poplars expressing a cysteine proteinase-inhibitor. Mol Breed 1(4): 319–328.
Lodja J, Gossrau R and Stoward PJ (1991) Proteases. In:Pearse AG (ed.), Histochemistry: Theoretical and Applied. Vol. 3 (pp. 281–335) Churchill Livingstone, London.
Losey JE, Rayor LS and Carter ME (1999) Transgenic pollen harms monarch larvae. Nature 399(6733): 214–214.
McManus MT, White DWR and McGregor PG (1994) Accumulation of a chymotrypsin inhibitor in transgenic tobacco can affect the growth of insect pests. Transgenic Res 3(1): 50–58.
Mochizuki A, Nishizawa Y, Onodera H, Tabei Y, Toki S, Habu Y, Ugaki M and Ohashi Y (1999) Transgenic rice plants expressing a trypsin inhibitor are resistant against rice stem borers, Chilo suppressalis. Entomol Exp Appl 93(2): 173–178.
Oberhause KSR, Prysby MD, Mattila HR, Stanley-Horn DE, Sears MK, Dively G, Olson E, Pleasants JM, Lam WKF and Hellmich RL (2001) Temporal and spatial overlap between monarch larvae and corn pollen. Proc Natl Acad Sci USA 98(21): 11913–11918.
Pham-Delegue MH, G irard C, Le Metayer M, Picard-Nizou AL, H ennequet C, Pons O and Jouanin L (2000) Long-term effects of soybean protease inhibitors on digestive enzymes, survival and learning abilities of honeybees. Entomol Exp Appl 95(1): 21–29.
Pitman EJG (1939) Biometrika 31: 9.
Raps A, Kehr J, Gugerli P, Moar WJ, Bigler F and Hilbeck A (2001) Immunological analysis of phloem sap of Bacillus thurigiensis corn and of the nontarget herbivore Rhopalosiphum padi (Homoptera: Aphididae) for the presence of Cry1Ab. Mol Ecol 10(2): 525–533.
Reed GL, Jensen AS, Riebe J, Head G and Duan JJ (2001) Transgenic Bt potato and conventional insecticides for Colorado potato beetle management: comparative efficacy and non-target impacts. Entomol Exp Appl 100(1): 89–100.
Schuler TH, Poppy GM and Denholm I (2000) Recommendations for the assessment of effects of GM crops on non-target organisms. In: Proceedings of the Brighton Crop Protection Council, British Crop Protection Council, Brighton, UK. Schuler TH, Denholm I, Jouanin L, Clark SJ, Clark AJ and Poppy GM (2001) Population-scale laboratory studies of the effect of transgenic plants on nontarget insects. Mol Ecol 10(7): 1845–1853.
Sears MK, Hellmich RL, Stanley-Horn DE, Oberhauser KS, Pleasants JM, Mattila HR, Siegfried BD and Dively GP (2001) Impact of Bt corn pollen on monarch butterfly populations: a risk assessment. Proc Natl Acad Sci USA 98(21): 11937–11942.
Stanley-Horn DE, Dively GP, Hellmich RL, Mattila HR, Sears MK, Rose R, Jesse L CH, Losey JE, Obrycki JJ and Lewis L (2001) Assessing the impact of Cry1Ab-expressing corn pollen on monarch butterfly larvae in field studies. Proc Natl Acad Sci USA 98(21): 11931–11936.
Thomas JC, Adams DG, Keppenne VD, W asmann CC, Brown JK, Kanost MR and Bohnert HJ (1995) Protease inhibitors of Manduca sexta expressed in transgenic cotton. Plant Cell Rep 14(12): 758–762.
Urwin PE, Atkinson HJ, W aller DA and McPherson MJ (1995) Engineered oryzacystatin-I expressed in transgenic hairy roots confers resistance to Globodera pallida. Plant J 8(1): 121–131.
Urwin PE, McPherson MJ and Atkinson HJ (1998) Enhanced transgenic plant resistance to nematodes by dual proteinase inhibitor constructs. Planta 204(4): 472–479.
Urwin PE, Troth KM, Zubko EI and Atkinson HJ (2001) Effective transgenic resistance to Globodera pallida in potato field trials. Mol Breed 8: 95–101.
Whitehead AG, N ichols AFJ and Senior JC (1991) Control of potato pale cyst-nematode, Globodera pallida, with a granular nematicide and partially resistant potatoes. Ann Appl Biol 118(3): 623–636.
Wraight CL, Zangerl AR, Carroll MJ and Berenbaum MR (2000) Absence of toxicity of Bacillus thuringiensis pollen to black swallowtails under field conditions. Proc Natl Acad Sci USA 97(14): 7700–7703.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cowgill, S., Atkinson, H. A Sequential Approach to Risk Assessment of Transgenic Plants Expressing Protease Inhibitors: Effects on Nontarget Herbivorous Insects. Transgenic Res 12, 439–449 (2003). https://doi.org/10.1023/A:1024215922148
Issue Date:
DOI: https://doi.org/10.1023/A:1024215922148