Abstract
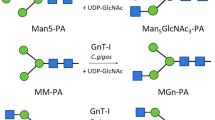
UDP-GlcNAc:α6-D-mannoside β1,2-N-acetylglucosaminyltransferase II (GnT II; EC 2.4.1.143) is a medial-Golgi resident enzyme that catalyses an essential step in the biosynthetic pathway leading from high mannose to complex N-linked oligosaccharides. Screening a cDNA library from Xenopus laevis ovary with a human GnT II DNA probe resulted in the isolation of two cDNA clones encoding two closely related GnT II isoenzymes, GnT II-A and GnT II-B. Analysis of the corresponding genomic DNAs revealed that the open reading frame of both X. laevis GnT II genes resides within a single exon. The GnT II-A gene was found to be transcriptionally active in all X. laevis tissues tested. In contrast, expression of the GnT II-B gene was detected only in a limited number of tissues. Both GnT II-A and GnT II-B exhibit a type II transmembrane protein topology with a putative N-terminal cytoplasmic tail of 9 amino acids followed by a transmembrane domain of 18 residues, and a C-terminal luminal domain of 405 residues. The two proteins differ at 28 amino acid positions within their luminal regions. Heterologous expression of soluble forms of the enzymes in insect cells showed that GnT II-A and GnT II-B are both catalytically active and exhibit similar specific activities. Both recombinant proteins are modified with N-linked oligosaccharides. N-terminal deletion studies demonstrated that the first 49 amino acid residues are not essential for proper folding and enzymatic activity of X. laevis GnT II. Published in 2003.
Similar content being viewed by others
References
Schachter H, Biochem Cell Biol 64, 163–81 (1986).
Kornfeld R, Kornfeld S, Annu Rev Biochem 54, 631–44 (1985).
Charuk JHM, Tan J, Bernardini M, Haddad S, Reithmeier RAF, Jaeken J, Schachter H, Eur J Biochem 230, 797–805 (1995).
Jaeken J, Schachter H, Carchon H, De Cock P, Coddeville B, Spik G, Arch Dis Child 71, 123–7 (1994).
Jaeken J, Spik G, Schachter H, Glycoproteins and Disease (Elsevier, Amsterdam, The Netherlands, 1996), pp. 457–67.
Tan J, Dunn J, Jaeken J, Schachter H, Am J Hum Genet 59, 810–7 (1996).
Schachter H, Jaeken J, Biochim Biophys Acta 1455, 179–92 (1999).
Wang Y, Tan J, Campbell RM, Ditto D, Le D, Schachter H, Marth JD, Glycobiology 10, 1131–2 (2000).
Wang Y, Tan J, Sutton-Smith M, Ditto D, Panico M, Campbell RM, Varki NM, Long JM, Jaeken J, Levison SR, Wynshaw BA, Morris HR, Le D, Dell A, Schachter H, Mart JD, Glycobiology 11, 1051–70 (2001).
Tan J, D'Agostaro GAF, Bendiak B, Reck F, Sarkar M, Squire JA, Leong P, Schachter H, Eur J Biochem 231, 317–28 (1995).
D'Agostaro GAF, Zingoni A, Moritz RL, Simpson RJ, Schachter H, Bendiak BJ, J Biol Chem 270, 15211–21 (1995).
Campbell R, Tan J, Schachter H, Bendiak B, Marth J, Glycobiology 7, 1050 (1997).
Leeb T, Kriegesmann B, Baumgartner BG, Klett C, Yerle M, Hameister H, Brenig B, Biochim Biophys Acta 1336, 361–6 (1997).
Chen S, Tan J, Reinhold VN, Spence AM, Schachter H, Biochim Biophys Acta 1573, 271–9 (2002).
Strasser R, Steinkellner H, Boren M, Altmann F, Mach L, Glössl J, Mucha J, Glycoconjugate J 16, 787–91 (1999).
Santacruz-Toloza L, Huang Y, John SA, Papazian, DM, Biochemistry 33, 5607–13 (1994).
Khanna R, Myers MP, Laine M, Papazian DM, J Biol Chem 276, 34028–34 (2001).
Roitsch T, Lehle L, Eur J Biochem 181, 733–9 (1989).
Cantor AB, Kornfeld S, Anal Biochem 205, 220–6 (1992).
Mucha J, Svoboda B, Fröhwein U, Strasser R, Mischinger M, Schwihla H, Altmann F, Hane W, Schachter H, Glössl J, Mach L, Glycobiology 11, 769–78 (2001).
Sarkar M, Pagny S, Unligil UM, Joziasse D, Mucha J, Glössl J, Schachter H, Glycoconjugate J 15, 193–7 (1998).
Chomczynski P, Sacchi N, Anal Biochem 162, 156–9 (1987).
Ausubel FM et al. (ed.), Current Protocols in Molecular Biology (John Wiley & Sons, Inc., 1994‐2002), Vol. 1, Ch. 2.
Unligil UM, Zhou S, Yuwaraj S, Sarkar M, Schachter H, Rini JM, EMBO J 19, 5269–80 (2000).
Altmann F, Anal Biochem 204, 215–9 (1992).
Altmann F, Kornfeld G, Dalik T, Staudacher E, Glössl J, Glycobiology 3, 619–25 (1993).
Kyte J, Doolittle RF, J Mol Biol 157, 105–32 (1982).
Breton C, Mucha J, Jeanneau C, Biochimie 83, 713–8 (2001).
Wiggins CAR, Munro S, Proc Natl Acad Sci USA 95, 7945–50 (1998).
Bisbee CA, Baker MA, Wilson AC, Haji-Azimi I, Fischberger M, Science 195, 785–7 (1977).
Amaya E, Offield MF, Grainger RM, Trends Genet 14, 253–5 (1998).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mucha, J., Svoboda, B., Kappel, S. et al. Two closely related forms of UDP-GlcNAc: α6-D-mannoside β1,2-N-acetylglucosaminyltransferase II occur in the clawed frog Xenopus laevis . Glycoconj J 19, 187–195 (2002). https://doi.org/10.1023/A:1024201824354
Issue Date:
DOI: https://doi.org/10.1023/A:1024201824354