Abstract
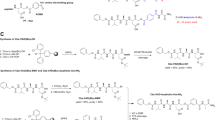
We recently demonstrated that tumor necrosis factor alpha activates caspase 6, which in turn cleaves transcription factor AP-2 alpha. We mapped the cleavage site at 19 amino acids from the N-terminus at the sequence aspartate-argenine-histidine-aspartate (DRHD). Mutating aspartic acid at position 19 abrogated the cleavage site. From these observations, we hypothesized that the DRHD peptide could act as a caspase 6 inhibitor. To test this hypothesis, the peptide zAsp(Ome)-Arg-His-Asp(Ome)-fluoromethyl ketone (zDRHDfmk) was synthesized. Here we show that zDRHDfmk inhibits TNFα-induced caspase 6 activity and apoptosis in breast cancer cells. When compared to other caspase inhibitors, zDRHDfmk inhibited caspase 6 activity more effectively than the general caspase inhibitor zVal-Ala-Lys(Ome)-fluoromethy ketone (zVADfmk) or the caspase 6 inhibitor zVal-Glu-Ile-Asp-(Ome)-fluoromethyl ketone (zVEIDfmk). However, it was less effective in inhibiting TNFα-induced apoptosis than zVADfmk or zVEIDfmk, presumably because caspase 6 is only one of at least three effector caspases, the others being caspase 3 and 7, that are active during caspase-dependent apoptosis. The discovery of this sequence-based caspase 6 inhibitor provides a new tool for studying caspase 6. More importantly, it could be used, in combination with other agents, as a drug to inhibit apoptosis in neurodegenrative diseases such as Alzheimer's, Parkinson and amyotrophic lateral sclerosis.
Similar content being viewed by others
References
Williams T, Admon A, Luscher B, Tijian R. Cloning and expression of AP-2, a cell-type specific transcription factor that activates inducible enhancer elements. Genes Dev 1988; 2: 1557-1569.
Moser M, Pscherer A, Roth C, et al. Enhanced apoptotic cell death of renal epithelial cells in mice lacking transcription factor AP-2?. Genes Dev 1997; 11: 1938-1948.
Oulad-Abdelghani M, Bouillet P, Chazaud C, Dolle P, Chambon P. AP-2.2: A novel AP-2-related transcription factor induced by retinoic acid during differentiation of P19 embryonal carcinoma cells. Exp Cell Res 1996; 225: 338-347.
Gaynor RB, Muchardt C, Xia YR, et al. Localization of the gene for the DNA-binding protein AP-2 to human chromosome 6p 22.3 pter. Genomics 1991; 10: 1100-1102.
Williamson JA, Bosher JM, Skinner JM, et al. Chromosomal mapping of the human and mouse homologues of two new members of the AP-2 family of transcription factors. Genomics 1996; 35: 262-264.
Zhao F, Satoda M, Licht JD, et al. Cloning and characterization of a novel mouse AP-2 transcription factor, AP-2delta, with unique DNA binding and transactivation properties. J Biol Chem 2002; 276: 40755-40760.
Schorle H, Meier P, Buchert M, Jaenisch R, Mitchell PJ. Transcription factor AP-2 essential for cranial closure and craniofacial development. Nature 1996; 381: 235-238.
Zhang J, Hagopian-Donaldson S, Serbedzija G, et al. Neural tube, skeletal and body wall defects in mice lacking transcription factor AP-2. Nature 1996; 381: 238-241.
Shen H, Wilke T, Ashique AM, et al. Chicken transcription factor AP-2: Cloning, expression and its role in outgrowth of facial prominences and limb buds. Dev Biol 1997; 188: 248-266.
Zeng Y-X, Soumasundaram K, El-Deiry W. AP-2 inhibits cancer cell growth and activates p21WAF1/CIP1 expression. Nature Genetics 1997; 15: 78-81.
van den Oord JJ, Vandeghinste N, De Ley M, et al. Bcl-2 expression in human melanocytes and melanocytic tumors. Am J Pathol 1994; 145: 294-300.
Aggarwal BB, Singh S, LaPushin R, Totpal K. Fas antigen signals proliferation of normal human diploid fibroblast and its mechanism is different from tumor necrosis factor receptor. FEBS Lett 1995; 364: 5-8.
Nyormoi O, Wang Z, Doan D, Ruiz M, McConkey D, Bar-Eli M. Transcription factor AP-2α is preferentially cleaved by caspase-6, and degraded by proteasome during TNF?-induced apoptosis in breast cancer cells. Mol Cell Biol 2001; 21: 4856-4867.
Dignam JD, Lebovitz RM, Roeder RG. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res 1983; 11: 1475-1489.
Nyormoi O, Schneider T, Smith RG. A large scale preparative gel electrophoresis separation of alpha 1 and alpha 2 subunits of the voltage-gated Ca2+ channel channel frochannel from rabbit skeletal muscle. Electrophoresis 1994; 15: 1186-1190.
Nyormoi O. Proteolytic activity in amyotrophic lateral sclerosis IgG preparations. Ann Neurol 1996; 40: 701-706.
Cohen GM. Caspases: The executioners of apoptosis. Biochem J 1997; 326: 1-16.
Earnshaw WC, Martins LM, Kaufmann SH. Mammalian caspases: Structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem 1999; 68: 383-424.
Peter ME, Heufelder AE, Hengartner MO. Advances in apoptosis research. Proc Natl Acad Sci USA 1997; 94: 12736-12737.
Roth KA, D'Sa C. Apoptosis and brain development. Ment Retard Dev Disabil Res Rev 2001; 7: 261-266.
Lacelle C, Xu S, Wang E. Identification of high caspase-3 mRNA expression as a unique signature profile for extremely old individuals. Mech Aging Dev 2002; 123: 1133-1144.
Pollack M, Phaneuf S, Dirks A, Leeuwenburgh C. The role of apoptosis in the normal aging brain, skeletal muscle, and heart. Ann NY Acad Sci 2000; 959: 93-107.
Springer JE, Nottingham SA, McEwen ML, Azbill RD, Jin Y. Caspase-3 apoptotic signaling following injury to the central nervous system. Clin Chem Lab Med 2001; 39: 299-307.
Kim M, Lee HS, LaForet G, et al. Mutant huntingtin expression in clonal striatal cells: Dissociation of inclusion formation and neuronal survival by caspase inhibition. J Neurosci 1999; 19: 964-973.
Rideout HJ, Stefanis L. Caspase inhibition: A potential therapeutic strategy in neurological diseases. Histol Histopathol 2001; 16: 895-908.
Hand CK, Rouleau GA. Familial amyotrophic lateral sclerosis. Muscle Nerve 2002; 25: 135-159.
Vukosavic S, Stefanis L, Jackson-Lewis V, et al. Delaying caspase activation by Bcl-2:Aclue to disease retardation in a transgenic mouse model of amyotrophic lateral sclerosis. J Neurosci 2000; 20: 9119-9125.
Ecder T, Melnikov VY, Stanley M, et al. Caspases, Bcl-2 proteins and apoptosis in autosomal-dominant polycystic kidney disease. Kidney Int 2002; 61: 1220-1230.
Garden GA, Budd SL, Tsai E, et al. Caspase cascades in human immunodeficiency virus-associated neurodegeneration. J Neurosci 2002; 22: 4015-4024.
Holcik M, Gibson H, Korneluk RG. XIAP: Apoptotic brake and promising therapeutic target. Apoptosis 2001; 6: 253-261.
Van Den Brande JM, Peppelenbosch MP, Van Deventer SJ. Treating Crohn's disease by inducing T lymphocyte apoptosis. Ann NY Acad Sci 2002; 973: 166-180.
Wellington CL, Hayden MR. Caspases and neurodegeneration: On the cutting edge of new therapeutic approaches. Clin Genet 2000; 57: 1-10.
Guttenplan N, Lee C, Frishman WH. Inhibition of myocardial apoptosis as a therapeutic target in cardiovascular disease prevention: Focus on caspase inhibition. Heart Dis 2001; 3: 313-318.
Salgado J, Garcia-Saez AJ, Malet G, Mingarro I, Perez-Paya E. Peptides in apoptosis research. J Pept Sci 2002; 8: 543-560.
Hirata H, Takahashi A, Kobayashi S, et al. Caspases are activated in a branched protease cascade and control distinct downstream processes in Fas-induced apoptosis. J Exp Med 1998; 187: 587-600.
Zhou Q, Snipas S, Orth K, Muzio M, Dixit VM, Slvesen GS. Target protease specificity of viral serpin CRM A: Analysis of five caspases. J Biol Biochem 1997; 272: 7797-7800.
Hay BA, Wolff T, Rubin GM. Expression of baculovirus p35 prevents cell death in Drosophila. Development 1994; 120: 2121-2129.
Yang YL, Li XM. The IAP family: Endogenous caspase inhibitors with multiple biological activities. Cell Res 2000; 10: 169-177.
Liu W, Staecker H, Stupak H, Malgrange B, Lefebvre P, Van De Water TR. Caspase inhibitors prevent cisplatin-induced apoptosis of auditory sensory cells. Neuroreport 1998; 9: 2609-2614.
Talanian RV, Quinlan C, Trautz S, et al. Substrate specificities of caspase family proteases. J Biol Chem 1997; 272: 9677-9682.
Thornberry NA, Rano TA, Peterson EP, et al. A combinatorial approach defines specificities of members of the caspase family and granzyme B. Functional relationships established for key mediators of apoptosis. Biol Chem 1997; 272: 17907-17911.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nyormoi, O., Wang, Z. & Bar-Eli, M. Sequence-based discovery of a synthetic peptide inhibitor of caspase 6. Apoptosis 8, 371–376 (2003). https://doi.org/10.1023/A:1024173018750
Issue Date:
DOI: https://doi.org/10.1023/A:1024173018750