Abstract
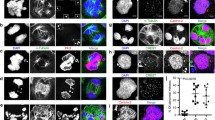
Epstein-Barr virus (EBV) is associated with a number of human malignancies. In vitro EBV infection transforms human lymphocytes into proliferating cell lines (EBV-lymphocytes). Etoposide, topoisomerase II inhibitor, induced apoptosis in EBV-lymphocytes as shown by expression of phosphatidylserine, loss of DNA and mitochondrial membrane potential, and cell shrinkage. In contrast, those cells, which had yet to display signs of apoptosis, grew to exceed their normal size. These EBV-lymphocytes had unusual cellular and nuclear morphology, higher mitochondrial membrane potential, increased expression of proteins and an amount of DNA that exceeded the maximum DNA content in normal EBV-lymphocytes by more than two-fold. Application of the caspase inhibitor Z-VAD-FMK in the presence of etoposide increased the numbers of such large cells. This data suggests that inhibition of topoisomerase II by etoposide does not inhibit DNA synthesis but rather overrides the G2/M check points of the cell cycle, resulting in cells growth over their genetically determined size. This may trigger apoptosis to eliminate cells, which failed to complete mitosis.
Similar content being viewed by others
References
Rickinson AB, Keiff E. Epstein-Barr virus. In: Fields BN, ed. Fields Virology. Philadelphia: Lippincott-Raven, 1996: 2397-2446.
Bornkamm GW, Hammerschmidt W. Molecular virology of Epstein-Barr virus. Phil Trans R Soc Lond B 2001; 356: 437-459.
Sinclair AJ, Fenton M, Delikat S. Interactions between Epstein-Barr virus and the cell cycle control machinery. Histol Histopathol 1998; 13: 461-467.
Brennan P. Signalling events regulating lymphoid growth and survival. Seminars Cancer Biol 2001; 11: 415-421.
Subramanian C, Knight JS, Robertson ES. The Epstein Barr nuclear antigen EBNA3C regulates transcription, cell transformation and cell migration. Frontiers Bioscie 2002; 7: D704-D716.
Frost V, Delikat S, Al-Mehairi S, Sinclair AJ. Regulation of p27 (KIP1) in Epstein-Barr virus-immortalized lymphoblastoid cell lines involves non-apoptotic caspase cleavage. J General Virol 2001; 82: 3057-3066.
Noguchi T, Ikeda K, Yamamoto K, et al. Antisense oligodeoxynucleotides to latent membrane protein 1 induce growth inhibition, apoptosis and Bcl-2 suppression in Epstein-Barr virus (EBV)-transformed B-lymphoblastoid cells, but not in EBV-positive natural killer cell lymphoma cells. Br J Haematol 2001; 114: 84-92.
Kenney JL, Guinness ME, Reiss M, Lacy J. Antisense to the Epstein-Barr virus (EBV)-encoded latent membrane protein 1 (LMP-1) sensitizes EBV-immortalized B cells to transforming growth factor-beta and chemotherapeutic agents. Intern J Cancer 2001; 91: 89-98.
Kobayashi K, Ratain MJ. Pharmacodynamics and long-term toxicity of etoposide. Cancer Chemotherapy Pharmacol 1994; 34: S64-S68.
Maia DM, Peace-Brewer AL. Chronic, active Epstein-Barr virus infection. Curr Opin Hematol 2000; 7: 59-63.
Su IJ. Etoposide (vp-16) inhibits epstein-barr virus determined nuclear antigen (ebna) synthesis. Br J Haematol 1995; 90: 972-973.
Kikuta H, Sakiyama Y. Etoposide (vp-16) inhibits epsteinbarr virus determined nuclear antigen (ebna) synthesis. Br J Haematol 1995; 90: 971-972.
Uno M, Tsuchiyama J, Moriwaki A, et al. In vitro induction of apoptosis for nasal angiocentric natural killer cell lymphomaderived cell line, NK-YS, by etoposide and cyclosporine A. Br J Haematol 2001; 113: 1009-1014.
Kaufmann SH. Cell death induced by topoisomerase-targeted drugs-more questions than answers. Biochim Biophys Acta 1998; 1400: 195-211.
Ishiyama K, Satoh S, Igarashi Y, et al. Flow cytometric analysis of the cell cycle of the leukemic cell lines treated with etoposide and cytosine arabinoside. Tohoku J Experim Med 1994; 174: 95-107.
Sugimoto K, Tamayose K, Takagi M, et al. Activation of an ataxia telangiectasia mutation-dependent intra-S-phase checkpoint by anti-tumour drugs in HL-60 and human lymphoblastoid cells. Br J Haematol 2000; 110: 819-825.
Cliby WA, Lewis KA, Lilly KK, Kaufmann SH. S phase and G(2) arrests induced by topoisomerase I poisons are dependent on ATR kinase function. J Biol Chem 2002; 277: 1599-1606.
Smith PJ, Soues S, Gottlieb T, et al. Etoposide-induced cell cycle delay and arrest-dependent modulation of dna topoisomerase ii in small-cell lung cancer cells. Br J Cancer 1994; 70: 914-921.
Inaba M, Mitsuhashi J, Kawada S, Nakano H. Different modes of cell-killing action between dna topoisomerase i and ii inhibitors revealed by kinetic analysis. Jap J Cancer Res 1994; 85: 187-193.
Pellicciari C, Bottone MG, Schaack V, Manfredi AA, Barni S. Etoposide at different concentrations may open different apoptotic pathways in thymocytes. Eur J Histochem 1996; 40: 289-298.
Dawkins JL, Hulme DJ, Brahmbhatt SB, Auer-Grumbach M, Nicholson GA. Mutations in SPTLC1, encoding serine palmitoyltransferase, long chain base subunit-1, cause hereditary sensory neuropathy type I. Nature Genetics 2001; 27: 309-312.
Vermes I, Haanen C, Reutelingsperger C. Flow cytometry of apoptotic cell death. J Immunol Methods 2000; 243: 167-190.
Darzynkiewicz Z. Acid-induced denaturation of DNA in situ as a probe for chromatin structure. Methods Cell Biol 1994; 41: 527-541.
Blatt NB, Glick GD. Signaling pathways and effector mechanisms pre-programmed cell death. Bioorg Med Chem 2001; 9: 1371-1384.
Dedov VN, Cox GC, Roufogalis BD. Visualization of mitochondria in living neurons with single and two-photon fluorescence laser scanning microscopy. Micron 2001; 32: 653-660.
Endresen PC, Prytz PS, Aarbakke J. A new flow cytometric method for discrimination of apoptotic cells and detection of their cell cycle specificity through staining of f-actin and dna. Cytometry 1995; 20: 162-171.
Uhlinger DJ, Carton JM, Argentieri DC, Damiano BP, D'Andrea MR. Increased expression of serine palmitoyltransferase (SPT) in balloon-injured rat carotid artery. Thrombosis Haemostasis 2001; 86: 1320-1326.
Mandon EC, Ehses I, Rother J, van Echten G, Sandhoff K. Subcellular localization and membrane topology of serine palmitoyltransferase, 3-dehydrosphinganine reductase, and sphinganine N-acyltransferase in mouse liver. J Biol Chem 1992; 267: 11144-11148.
Merrill AH, Schmelz EM, Dillehay DL, et al. Sphingolipids-the enigmatic lipid class-biochemistry, physiology, and pathophysiology. Toxicol Applied Pharmacol 1997; 142: 208-225.
Halicka HD, Seiter K, Feldman EJ, et al. Cell cycle specificity of apoptosis during treatment of leukaemias. Apoptosis 1997; 2: 25-39.
Pokrovskaja K, Okan I, Kashuba E, et al. Epstein-Barr virus infection and mitogen stimulation of normal B cells induces wild-type p53 without subsequent growth arrest or apoptosis. J General Virol 1999; 80: 987-995.
Dedov VN, Armati PJ, Roufogalis BD. Three-dimensional organization of mitochondrial clusters in regenerating dorsal root ganglion (DRG) neurons from neonatal rats: Evidence for mobile mitochondrial pools. J Periph Nerve System 2000; 5: 3-10.
King MA, Radicchi-Mastroianni MA. Effects of caspase inhibition on camptothecin-induced apoptosis of HL-60 cells. Cytometry 2002; 49: 28-35.
Smits VAJ, Medema RH. Checking out the G2/M transition. Biochim Biophys Acta 2001; 1519: 1-12.
Bell SP, Dutta A. DNA replication in eukaryotic cells. Annu Rev Biochem 2002; 71: 333-374.
Polymenis M, Schmidt EV. Coordination of cell growth with cell division. Curr Opin Genetics Develop 1999; 9: 76-80.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dedov, V.N., Dedova, I.V. & Nicholson, G.A. Inhibition of topoisomerase II overrides the G2/M check points of the cell cycle in EBV-lymphocytes. Apoptosis 8, 399–406 (2003). https://doi.org/10.1023/A:1024133304637
Issue Date:
DOI: https://doi.org/10.1023/A:1024133304637