Abstract
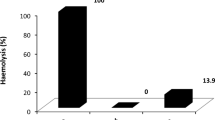
Unconjugated bilirubin binds to erythrocytes, eliciting crenation, lipid elution and hemolysis. The present work attempts to establish the role of acidosis on bilirubin-induced toxicity to human erythrocytes. To this end, pH values ranging from 7.0–8.0 were used to induce a different representation of acid and anionic bilirubin species, respectively. Erythrocytes from healthy donors were incubated with bilirubin and albumin (3:1, molar ratio), during 4 h. Erythrocyte-bound bilirubin was evaluated by albumin or chloroform extraction in an attempt to assess either mono- and dianion bilirubin adsorbed on the cell surface or colloidal aggregates, respectively. Cytotoxicity indicators, such as the morphological index, and the extent of phospholipids and hemoglobin release were also determined. The results showed that as pH drops from 8.0–7.0, less bilirubin is removed by albumin and more become recovered by chloroform. The data corroborate the predominance of anionic and non-aggregated bilirubin species at pH 8.0 with dimers and precipitates occurring at 7.0. In accordance, crenation and cell lysis were four times increased at acidic pH. In contrast, elution of phospholipids was 1.5 times less evident at the same pH, thus suggesting that formation of bilirubin complexes with membrane phospholipids may have contributed to prevent their release. In conclusion, both anionic and acid bilirubin species interact with human erythrocytes leading to cytotoxic alterations that may determine definitive lesions. Nevertheless, increased vulnerability to crenation and hemolysis are more likely to occur in acidic conditions pointing to the bilirubin precipitates as the main candidates of bilirubin-induced toxicity to erythrocytes.
Similar content being viewed by others
References
Jacobsen J: Binding of bilirubin to human serum albumin — determination of the dissociation constants. FEBS Lett 5: 112-114, 1969
Bratlid D, Fog J: The binding capacity of human albumin for bilirubin and its significance in the pathogenesis of kernicterus. Scand J Clin Lab Invest 25: 257-261, 1970
Brodersen R: Binding of bilirubin to albumin. CRC Crit Rev Clin Lab Sci 11: 305-401, 1980
Kim MH, Yoon JJ, Sher J, Brown AK: Lack of predictive indices in kernicterus: a comparison of clinical and pathologic factors in infants with or without kernicterus. Pediatrics 66: 852-858, 1980
Perlman JM, Rogers BB, Burns D: Kernicteric findings at autopsy in two sick near term infants. Pediatrics 99: 612-615, 1997
Harris RC, Lucey JF, MacLean JR: Kernicterus in premature infants associated with low concentrations of bilirubin in the plasma. Pediatrics 21: 875-884, 1958
Stern L, Denton RL: Kernicterus in small premature infants. Pediatrics 35: 483-485, 1965
Alayoff A, Kapitulnik J, Konijn A, Kaufmann NA, Blondheim SH: Bilirubin binding capacity of albumin isolated from cord-blood serum is less than that from serum of adults. Clin Chem 26: 738-740, 1980
Ritter DA, Kenny JD: Influence of gestational age on cord serum bilirubin binding studies. J Pediatr 106: 118-121, 1985
Gourley GR: Bilirubin metabolism and kernicterus. Adv Pediatr 44: 173-229, 1997
Brodersen R: Binding of bilirubin to albumin and tissues. In: M. Monset-Couchard, A. Minkowski (eds). Phsysiological and Biochemical Basis for Perinatal Medicine. Samuel Z. Levine Conference, First International Meeting, Paris. S. Karger, Basel, 1979, pp 144-152.
De Vries LS, Lary S, Dubowitz LMS: Relationship of serum bilirubin levels to ototoxicity and deafness in high-risk low-birth-weight infants. Pediatrics 76: 351-354, 1985
Wennberg RP, Gospe SM, Rhine WD, Seyal M, Saeed D, Sosa G: Brainstem bilirubin toxicity in the newborn primate may be promoted and reversed by modulating PCO2. Pediatr Res 34: 6-9, 1993
Nelson T, Jacobsen J, Wennberg RP: Effect of pH on the interaction of bilirubin with albumin and tissue culture cells. Pediatr Res 8: 963-967, 1974
Ostrow JD, Mukerjee P, Tiribelli C: Structure and binding of unconjugated bilirubin: Relevance for physiological and pathophysiological function. J Lipid Res 35: 1715-1737, 1994
Athar H, Ahmad N, Tayyab S, Qasim MA: Use of fluorescence enhancement technique to study bilirubin-albumin interaction. Int J Biol Macromol 25: 353-358, 1999
Kashiwamata S, Suzuki F, Semba K: Affinity of young rat cerebral slices for bilirubin and some factors influencing its transfer to the slices. Jpn J Exp Med 50: 303-311, 1980
Wennberg RP: The importance of free bilirubin acid salt in bilirubin uptake by erythrocytes and mitochondria. Pediatr Res 23: 443-447, 1988
Amit Y, Fedunec S, Thomas PD, Poznansky MJ, Schiff D: Bilirubinneural cell interaction: Characterization of initial cell surface binding leading to toxicity in the neuroblastoma cell line N-115. Biochim Biophys Acta 1055: 36-42, 1990
Brodersen R, Stern L: Deposition of bilirubin acid in the central nervous system — a hypothesis for the development of kernicterus. Acta Paediatr Scand 79: 12-19, 1990
Silva R, Mata LR, Gulbenkian S, Brito MA, Tiribelli C, Brites D: Inhibition of glutamate uptake by unconjugated bilirubin in cultured cortical rat astrocytes: Role of concentration and pH. Biochem Biophys Res Commun 265: 67-72, 1999
Rodrigues CMP, Solá S, Silva R, Brites D: Bilirubin and amyloid-β peptide induce cytochrome c release through mitochondrial membrane permeabilization. Mol Med 6: 936-946, 2000
Silva R, Rodrigues CMP, Brites D: Bilirubin-induced apoptosis in neural cells is aggravated by chenodeoxycholic acid but prevented by ursodeoxycholic acid. J Hepatol 34: 402-408, 2001
Silva RFM, Mata LR, Gulbenkian S, Brites D: Endocytosis in rat cultured astrocytes is inhibited by unconjugated bilirubin. Neurochem Res 26: 791-798, 2001
Rodrigues CMP, Solá S, Brito MA, Brites D, Moura JJG: Bilirubin directly disrupts membrane lipid polarity and fluidity, protein order, and redox status in rat mitochondria. J Hepatol 36: 335-341, 2002
Watson D: The absorption of bilirubin by erythrocytes. Clin Chim Acta 7: 733-734, 1962
Bratlid D: Bilirubin binding by human erythrocytes. Scand J Clin Lab Invest 29: 91-97, 1972
Tayyab S, Ali MK: Binding of bilirubin to mammalian erythrocytes. Comp Biochem Physiol 118B: 97-103, 1997
Brito MA, Silva R, Tiribelli C, Brites D: Assessment of bilirubin toxicity to erythrocytes. Implication in neonatal jaundice management. Eur J Clin Invest 30: 239-247, 2000
Bratlid D: The effect of pH on bilirubin binding to human erythrocytes. Scand J Clin Lab Invest 29: 453-459, 1972
Rashid H, Ali MK, Tayyab S: Effect of pH and temperature on the binding of bilirubin to human erythrocyte membranes. J Biosci 25: 157-161, 2000
Kaul R, Bajpai VK, Shipstone AC, Kaul HK, Krishna Murti CR: Bilirubin-induced erythrocyte membrane cytotoxicity. Exp Mol Pathol 34: 290-298, 1981
Brito MA, Silva RM, Matos DC, da Silva AT, Brites DT: Alterations of erythrocyte morphology and lipid composition by hyperbilirubinemia. Clin Chim Acta 249: 149-165, 1996
Brites D, Silva R, Brito A: Effect of bilirubin on erythrocyte shape and haemolysis, under aggregating or non-aggregating conditions, and correlation with cell age. Scand J Clin Lab Invest 57: 337-350, 1997
Brito MA, Silva RFM, Brites D: Bilirubin induces loss of membrane lipids and exposure of phosphatidylserine in human erythrocytes. Cell Biol Toxicol 18: 181-192, 2002
McDonagh AF, Assissi F: Commercial bilirubin: A trinity of isomers. FEBS Lett 18: 315-317, 1971
Hansen TWR: Acute management of extreme neonatal jaundice — the potential benefits of intensified phototherapy and interruption of enterohepatic bilirubin circulation. Acta Paediatr 86: 843-846; 1997
Jansen PL: Diagnosis and management of Crigler-Najjar syndrome. Eur J Pediatr 158(suppl 2): S89-S94, 1999
Kancko K, Takei Y, Aoki T, Ikeda S, Matsunami H, Lynch S: Bilirubin adsorption therapy and subsequent liver transplantation cured severe bilirubin encephalopathy in a long-term survival patient with Crigler-Najjar disease type I. Intern Med 39: 871-872, 2000
Hsu RC, Kanofsky JR, Yachnin S: The formation of echinocytes by the insertion of oxygenated sterol compounds into red cell membranes. Blood 56: 109-117, 1980
Fujii T, Sato T, Tamura A, Wakatsuki M, Kanaho Y: Shape changes of human erythrocytes induced by various amphipathic drugs acting on the membrane of the intact cells. Biochem Pharmacol 28: 613-620, 1979
Moreau-Clevede J, Pays M: Détermination de la bilirubine érythrocytaire. Ann Biol Clin 37: 95-101, 1979
Brites D, Silveira C, Pays M: Determinação da bilirrubina em patologia neonatal — modificação do método de Pays para concentrações de 1 a 10 mg por 100 ml. Rev Port Farm 28: 253-262, 1978
Dodge JT, Mitchell C, Hanahan DJ: The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys 100: 119-129, 1963
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ: Protein measurements with the Folin phenol reagent. J Biol Chem 193: 265-275, 1951
McDonagh AF: Bile pigments: Bilatrienes and 5,15 biladienes. In: D. Dolphin (ed). The Porphyrins. Academic Press, New York, 1979, pp 294-491
Malheiros SVP, Brito MA, Brites D, Meirelles NC: Membrane effects of trifluoperazine, dibucaine and praziquantel on human erythrocytes. Chem Biol Interact 126: 79-95, 2000
Fiske CH, Subbarow Y: The colorimetric determination of phosphorus. J Biol Chem 66: 375-400, 1925
Sato H, Kashiwamata S: Interaction of bilirubin with human erythrocyte membranes. Biochem J 210: 489-496, 1983
Sugita K, Sato T, Nakajima H: Effects of pH and hypoglicemia on bilirubin cytotoxicity in vitro. Biol Neonate 52: 22-25, 1987
Eriksen EF, Danielsen H, Brodersen R: Bilirubin-liposome interaction. Binding of bilirubin dianion, protonization, and aggregation of bilirubin acid. J Biol Chem 256: 4269-4274, 1981
Cestaro B, Cervato G, Ferrari S, Di Silvestro G, Monti D, Manitto P: Interaction of bilirubin with small unilamellar vesicles of dipalmitoyl-phosphatidylcholine. Ital J Biochem 32: 318-329, 1983
Vázquez J, García-Calvo M, Valdivieso F, Mayor F, Mayor F Jr: Interaction of bilirubin with the synaptosomal plasma membrane. J Biol Chem 263: 1255-1265, 1988
Brito MA: Interacção da bilirrubina com o eritrócito. Espécies moleculares envolvidas, estádios de toxicidade e comportamento da célula fetal. Ph.D. Thesis, University of Lisbon, 2001
Brito MA, Brondino CD, Moura JJG, Brites D: Effects of bilirubin molecular species on membrane dynamic properties of human erythrocyte membranes: A spin label electron paramagnetic resonance spectroscopy study. Arch Biochem Biophys 387: 57-65, 2001
Cheung WH, Sawitsky A, Isenberg HD: The effect of bilirubin on the mammalian erythrocyte. Transfusion 6: 475-476, 1966
Vinardell MP: Characteristics of interaction between amphiphiles and membranes. Trends Comp Biochem Physiol 2: 73-82, 1996
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Brito, M.A., Brites, D. Effect of acidosis on bilirubin-induced toxicity to human erythrocytes. Mol Cell Biochem 247, 155–162 (2003). https://doi.org/10.1023/A:1024111613327
Issue Date:
DOI: https://doi.org/10.1023/A:1024111613327