Abstract
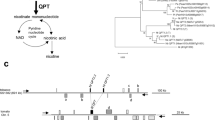
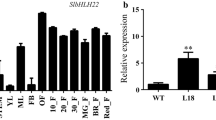
The genomic organization of two extracellular invertase genes from tomato (Lin5 and Lin7), which are linked in a direct tandem repeat, and their tissue-specific and hormone-inducible expression are shown. Transient expression analysis ofLin5 promoter sequences fused to the β-glucuronidase (GUS) reporter gene (uidA) demonstrates a specific expression of Lin5during tomato fruit development. A Lin5 promoter fragment was fused to the truncated nos promoter to analyse hormone induction via GUS reporter gene activity in transiently transformed tobacco leaves. A specific up-regulation of GUS activity conferred by this Lin5 promoter fragment in response to gibberellic acid (GA), auxin and abscisic acid (ABA) treatment was observed, indicating a critical role of the regulation of Lin5 by phytohormones in tomato flower and fruit development. In situ hybridization analysis of Lin7 shows a high tissue-specific expression in tapetum and pollen. These results support an important role for Lin5 and Lin7 extracellular invertases in the development of reproductive organs in tomato and contribute to unravel the underlying regulatory mechanisms.
Similar content being viewed by others
References
Bate, N., Spurr, C., Foster, G.D. and Twell, D. 1996. Maturationspecific translational enhancement mediated by the 5? UTR of a late pollen transcript. Plant J. 10: 613–623.
Berleth, T. and Sachs, T. 2001. Plant morphogenesis: long-distance coordination and local patterning. Curr. Opin. Plant Biol. 4: 57–62.
Bradford, M.M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254.
Clément, C., Burrus, M. and Audran, J.-C. 1996. Floral organ growth and carbohydrate content during pollen development in Lilium. Am. J. Bot. 83: 459–469.
Eschrich, W. 1980. Free space invertase, its possible role in Phloem unloading. Ber. Dtsch. Bot. Ges. 93: 363–378.
Evert, R.F.1982. Sieve-tube structure in relation to function. Bioscience 32: 789–795.
Fridman, E., Liu, Y.S., Carmel-Goren, L., Gur, A., Shoresh, M., Pleban, T., Eshed, Y. and Zamir, D. 2002. Two tightly linked QTLs modify tomato sugar content via different physiological pathways. Mol. Genet. Genomics 266: 821–826.
Godt, D.E. and Roitsch, T. 1997. Regulation and tissue-specific distribution of mRNA for three extracellular invertase isoenzymes of tomato suggests an important function in establishing and maintaining sink metabolism. Plant Physiol. 115: 273–282.
Goetz, M. and Roitsch, T. 1999. The different pH optima and substrate specificities of extracellular and vacuolar invertases are determined by a single amino acid substitution. Plant J. 20: 707–711.
Goetz, M., Godt, D.E. and Roitsch T. 2000. Tissue-specific induction of the mRNA for an extracellular invertase isoenzyme of tomato by brassinosteroids suggests a role of steroid hormones in asssimilate partitioning. Plant J. 22: 515–522.
Goetz, M., Godt, D.E., Guivarch, A., Kahmann, U., Chriqui, D. and Roitsch, T. 2001. Induction of male sterility in plants by metabolic engineering of the carbohydrate supply. Proc. Natl. Acad. Sci. USA 98: 6522–6527.
Grotewold, E., Drummond, B.J., Bowen, B. and Peterson, T. 1994. The myb-homologous P gene controls phlobaphene pigmentation in maize floral organs by directly activating a flavonoid biosynthetic gene subset. Cell 76: 543–553.
Gubler, F., Kalla, R., Roberts, J.K., and Jacobsen, J.V. 1995. Gibberellin-regulated expression of a myb gene in barley aleurone cells: evidence for myb transactivation of a high-pI α-amylase gene promoter. Plant Cell 7: 1879–1891.
Joshi, C. P. 1987. An inspection of the domain between putative TATA box and translation site in 79 plant genes. Nucl. Acid. Res. 15: 6643–6653.
Kim, J.-Y, Mahé, A., Guy, S., Brangeon, J., Roche, O., Chourey, P.S. and Prioul, J.-L. 2000. Characterization of two members of the maize gene family, Incw3 and Incw4, encoding cell-wall invertases. Gene 245: 89–102.
Linden, J.C., Ehness, R. and Roitsch, T. 1996. Ethylene regulation of apoplastic invertase expression in autotrophic cells of Chenopodium rubrum. Plant Growth Reg. 19: 219–222.
Lorenz, K., Lienhard, S. and Sturm, A. 1995. Structural organization and differential expression of carrot α-fructofuranosidase genes: identification of a gene coding for a flower bud-specific isoenzyme. Plant Mol. Biol. 28: 189–194.
Maddison, A.L., Hedeley, P.E., Meyer, R.C., Aziz, N., Davidson, D. and Machray, G.C. 1999. Expression of tandem invertase genes associated with sexual and vegetative growth cycles in potato. Plant Mol. Biol. 41: 741–751.
Ranwala, A.P., and Miller, W.B. 1998. Sucrose-cleaving enzymes and carbohydrate pools in Lilium longiflorum floral organs. Physiol. Plant 103: 541–550.
Rebers, M., Kaneta, T., Kawaide, H., Yamaguchi, S., Yang, Y.-Y., Imai, R., Sekimoto, H. and Kamiya, Y. 1999. Regulation of gibberellin biosynthesis genes during flower and early fruit development of tomato. Plant J. 17: 341–250.
Richards, D.E., King, K.E., Ait-ali, T. and Harberd, N.P. 2001. How gibberellin regualtes plant growth and development: a molecular genetic analysis of gibberellin signaling. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52: 67–88.
Roitsch, T., Ehness, R., Götz, M., Hause, B., Hofmann, M. and Sinha, A. 2000. Regulation and function of extracellular invertase from higher plants in relation to assimilate partitioning, stress responses and sugar signalling. Aust. J. Plant Physiol. 27: 815–825.
Shen, Q. and Ho, T.-H.D. 1995. Functional dissection of an abscisic acid (ABA)-inducible gene reveals two independent ABAresponsive complexes each containing a G-box and a novel cis-acting element. Plant Cell 7: 295–307.
Solano, R., Nieto, C., Avila, J., Canas, L., Diaz, I. and Paz-Ares, J. 1995. Dual DNA-binding specificity of a petal epidermis-specific MYB transcription factor (MYB.Ph3) from Petunia hybrida. EMBO J. 14: 1773–1784.
Spolaore, S., Trainotti, L. and Casadoro, G. 2001. A simple protocol for transient gene expression in ripe fleshy fruit mediated by Agrobacterium. J. Exp. Bot. 52: 845–850.
Szabados, L., Charrier, B., Kondorosi, A., de Bruijn, F.J. and Ratet, P. 1995. New plant promoter and enhancer testing vectors. Mol. Breed. 1: 419–423.
Twell, D., Yamaguchi, J., Wing, R.A., Ushiba, J. and McCormick, S. 1991. Promotor analysis of genes that are coordinately expressed during pollen development reveals pollen-specific enhancer sequences and shared regulatory elements. Genes Dev. 5: 496–507.
Tymowska-Lalanne, Z. and Kreis, M. 1998a. The plant invertases: physiology, biochemistry and molecular biology. Adv. Bot. Res. 28: 71–117.
Tymowska-Lalanne, Z. and Kreis, M. 1998 b. Expression of the Arabidopsis thaliana invertase gene family. Planta 210: 259–265.
Tymowska-Lalanne, Z., Schwebel-Dugué, N., Lecharny, A. and Kreis, M. 1996. Expression and cis-acting elements of the At?fruct1 gene from Arabidopsis thaliana endoding a cell wall invertase. Plant Physiol. Biochem. 43: 431–442.
Ulmasov, T., Hagen, G. and Guilfoyle, T.J. 1999. Activation and repression of transcription by auxin-response factors. Proc. Natl. Acad. Sci. USA 96: 5844–5849.
Weber, H., Borisjuk, L., and Wobus, U. 1996. Controlling seed development and seed size in Vicia faba: a role for seed coat-associated invertases and carbohydrate state. Plant J. 10: 823–834.
Weil, M. and Rausch, T. 1990. Cell wall invertase in tobacco crown gall cells. Plant Physiol. 94: 1575–1581.
Weterings, K., Schrauwen, J., Willems, G. and Twell, D. 1995. Functional dissection of the promoter of the pollen-specific gene Ntp303 reveals a novel pollen-specific and conserved cisregulatory element. Plant J. 8: 55–63.
Wu, L.-L., Mitchell, J.P., Cohn, N.S. and Kaufman P.B. 1993. Gibberellin (GA3) enhances cell wall invertase activity and mRNA levels in elongating dwarf pea (Pisum sativum) shoots. Int. J. Plant Sci. 154: 280–289.
Xu, J., Avigne,W.T., McCarty, D.R. and Koch, K.E. 1996. A similar dichotomy of sugar modulation and developmental expression affects both paths of sucrose metabolism: evidence from maize invertase gene family. Plant Cell 8: 1209–1220.
Yinong, Y., Rugana, L., and Min, Q. 2000. In vivo analysis of plant promoters and transcription factors by agroinfiltration of tobacco leaves. Plant J. 22: 543–551.
Zou, J.T., Zhan, X.Y., Wu, H.M., Wang, H. and Cheung, A.Y. 1994. Characterization of a rice pollen-specific gene and its expression. Am. J. Bot. 81: 552–561.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Proels, R., Hause, B., Berger, S. et al. Novel mode of hormone induction of tandem tomato invertase genes in floral tissues. Plant Mol Biol 52, 191–201 (2003). https://doi.org/10.1023/A:1023973705403
Issue Date:
DOI: https://doi.org/10.1023/A:1023973705403