Abstract
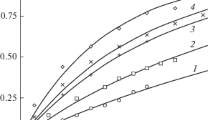
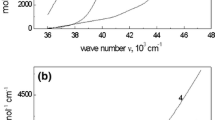
The copper and zinc dissolution in phosphate solutions is studied over wide pH range (from 4.5 to 11.7). The cathodic reaction rates are shown to depend on the pH of the solutions; they are minimum at a pH of 9.5 for zinc and 11.7 for copper. Phosphate directly participates in the cathodic process on zinc because of the reduction of phosphate to phosphite anions. Thermodynamic calculations, corroborated by electrochemical studies and analyses of the electrode surfaces by different methods, showed that zinc passivates itself by the deposition of either ZnO, Zn(OH)2, Zn(H2PO4)2, and ZnHPO4, in the solutions of low acidity, or ZnO, Zn(OH)2, ZnHPO4, and Zn3(PO4)2 at a pH of 9.5 to 11.7. On copper, the passive films are formed of CuH2PO4 and CuHPO4 in weakly acid phosphate solutions or of CuO, Cu(OH)2, CuHPO4, and Cu3(PO4)2 at a pH of 9.5 to 11.7.
Similar content being viewed by others
REFERENCES
Kuznetsov, Yu.I., Rozenfel'd, I.L., and Podgornova, L.P., Zashch. Met., 1978, vol. 14, no. 5, p. 561.
Kuznetsov, Yu.I., Rozenfel'd, I.L., Podgornova, L.P., and Balashova, N.N., Elektrokhimiya, 1978, vol. 14, no. 12, p. 1869.
Kuznetsov, Yu.I. and Podgornova, L.P., Zashch. Met., 1983, vol. 19, no. 1, p. 98.
Kreshkov, A.P., Osnovy analiticheskoi khimii (Fundamentals of Analytical Chemistry), Moscow: Khimiya, 1970, vol. 1, p. 472.
Analiticheskaya khimiya elementov. Fosfor (Analytical Chemistry of Elements: Phosphorus), Moscow: Nauka, 1974, p. 218.
Latimer, W.M., The Oxidation States of Elements and Their Potentials in Aqueous Solutions, New York, 1952.
Maslikova, M.A. and Chemodanov, D.I., Izv. Akad. Nauk SSSR, Neorg. Mater., 1971, vol. 7, no. 10, p. 1773.
Varenko, E.S. and Galushko, V.P., Zashch. Met., 1973, vol. 9, no. 1, p. 103; no. 4, p. 460.
Vvedenskii, A.V. and Marshakov, I.K., Zashch. Met., 1983, vol. 19, no. 1, p. 79; no. 2, p. 282.
Kiss, L. and Varsanyi, L.M., Acta Chim. Acad. Sci. Hung., 1974, vol. 81, no. 1, p. 61.
Kuz'menko, A.L., Spravochnik po obshchei i neorganicheskoi khimii (Handbook of General and Inorganic Chemistry), Minsk: Vysshaya Shkola, 1974, p. 144.
Lur'e, Yu.Yu., Spravochnik po analiticheskoi khimii (Handbook of Analytical Chemistry), Moscow: Khimiya, 1979, p. 95.
Podgornova, L.P., Gorodetskii, A.E., Kuznetsov, Yu.I., and Kolesnichenko, A.A., Zashch. Met., 1980, vol. 16, no. 4, p. 500.
Dobos, D., Electrochemical Data, Budapest: Akad. Kiado, 1978, p. 365.
Wagner, C.D., Riggs, W.M., Davis, L.E., et al., Handbook of X-ray Photoelectron Spectroscopy, New York: Perkin-Elmer, 1979, p. 251.
Pourbaix, M., Atlas d'equilibres electrochimique a 25ºC, Paris: Gauthier-Vilars, 1963, p. 644.
Mikhailovskii, Yu.N. and Popova, V.M., Zashch. Met., 1984, vol. 20, no. 2, p. 204.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Podgornova, L.P., Kuznetsov, Y.I. & Gavrilova, S.V. On the Zinc and Copper Dissolution in Phosphate Solutions. Protection of Metals 39, 217–221 (2003). https://doi.org/10.1023/A:1023954817961
Issue Date:
DOI: https://doi.org/10.1023/A:1023954817961